Sol-Gel Synthesis of Metal Oxide Photocatalysts: A Comprehensive Guide from Fundamentals to Advanced Biomedical Applications
This article provides a comprehensive examination of the sol-gel method for synthesizing advanced metal oxide photocatalysts, tailored for researchers and drug development professionals.
Sol-Gel Synthesis of Metal Oxide Photocatalysts: A Comprehensive Guide from Fundamentals to Advanced Biomedical Applications
Abstract
This article provides a comprehensive examination of the sol-gel method for synthesizing advanced metal oxide photocatalysts, tailored for researchers and drug development professionals. It explores the foundational principles of sol-gel chemistry and its advantages for creating tailored nanostructures. The scope extends to practical synthesis protocols, characterization techniques, and optimization strategies to overcome common challenges like electron-hole recombination and limited visible-light absorption. By presenting validation frameworks and comparative performance analysis of various metal oxide systems, including TiO2, ZnO, and their composites, this guide serves as a critical resource for developing efficient photocatalytic platforms for environmental remediation, drug discovery, and clinical applications.
Sol-Gel Chemistry and Metal Oxide Photocatalysis: Fundamental Principles and Design Opportunities
Fundamental Chemical Principles
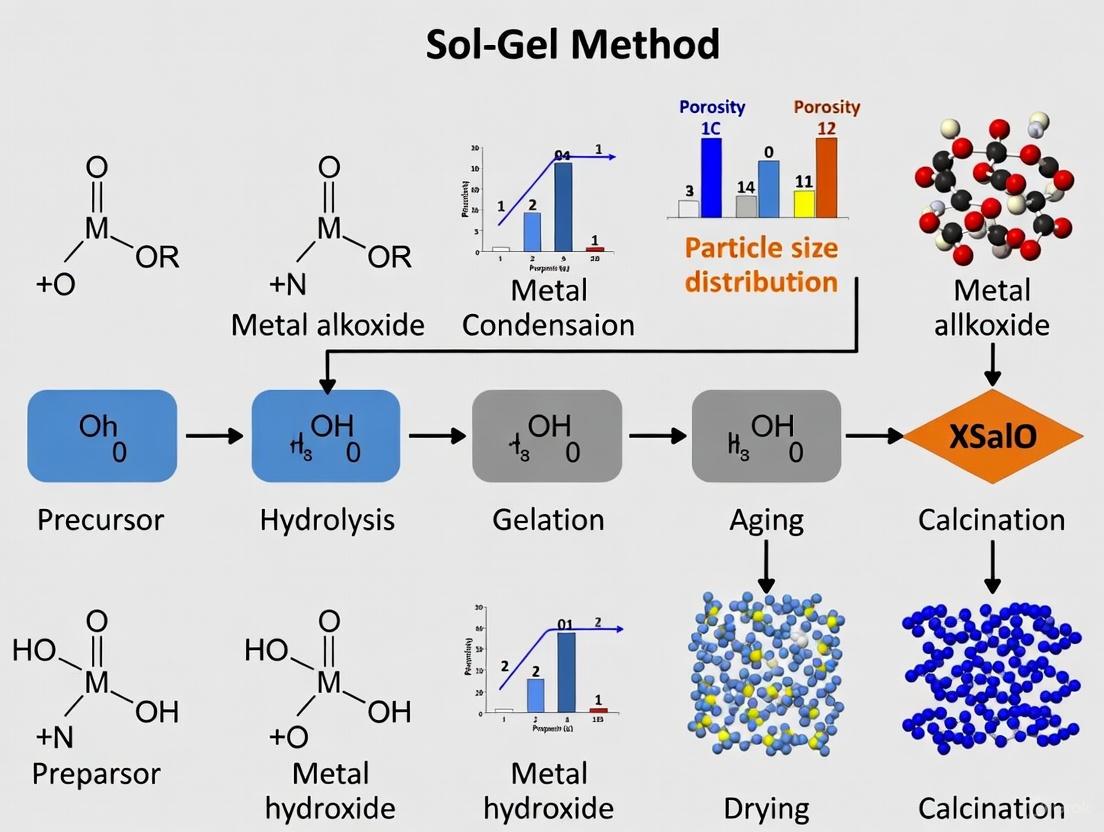
Sol-gel processing is a versatile wet-chemical method for fabricating metal oxide networks through a series of controlled hydrolysis and polycondensation reactions starting from molecular precursors [1] [2]. This bottom-up approach enables the transformation of a colloidal solution (sol) into an integrated solid network (gel) spanning various dimensionalities from discrete nanoparticles to continuous monolithic structures [2] [3].
The process fundamentally involves connecting metal centers through oxo (M-O-M) or hydroxo (M-OH-M) bridges, generating metal-oxo or metal-hydroxo polymers in solution [2]. The most commonly employed precursors are metal alkoxides, with tetraethylorthosilicate (TEOS) and titanium tetraisopropoxide (TTiP) being frequently used for silica and titania systems, respectively [1] [4].
Hydrolysis Mechanisms
Hydrolysis initiates the sol-gel process through nucleophilic attack of water molecules on metal alkoxide precursors. This reaction replaces alkoxide groups (OR) with hydroxyl groups (OH) [2] [5]:
General Hydrolysis Reaction: M(OR)₄ + H₂O → M(OR)₃(OH) + ROH
The rate and extent of hydrolysis are critically influenced by pH, water-to-precursor ratio, and the electronegativity of the metal center [5]. More electrophilic metal centers (e.g., Ti, Zn) undergo hydrolysis more readily than silicon, often necessitating modified precursors or reaction controls for multicomponent systems [4] [6].
Polycondensation Mechanisms
Following hydrolysis, polycondensation reactions create the metal oxide network through the formation of oxo-bridges, liberating water (aqua) or alcohol (alcoxo) as byproducts [1] [2]:
Water-Forming Condensation: M–OH + HO–M → M–O–M + H₂O
Alcohol-Forming Condensation: M–OR + HO–M → M–O–M + ROH
These condensation reactions proceed through SNâ‚‚-type nucleophilic substitution mechanisms, where the rate depends on the steric hindrance around the metal center and the catalyst employed [5]. The growing polymers eventually cross-link to form an extensive three-dimensional network, marking the gel point where the viscosity increases sharply and the system solidifies [1].
Table 1: Key Reactions in Sol-Gel Processing
| Reaction Type | General Equation | Products | Key Influencing Factors |
|---|---|---|---|
| Hydrolysis | M(OR)₄ + nH₂O → M(OR)₄₋ₙ(OH)ₙ + nROH | Partially hydrolyzed precursor, alcohol | pH, water concentration, catalyst type, metal electronegativity |
| Water-Forming Condensation | M–OH + HO–M → M–O–M + H₂O | Oxo-bridge, water | pH, temperature, metal reactivity |
| Alcohol-Forming Condensation | M–OR + HO–M → M–O–M + ROH | Oxo-bridge, alcohol | Steric hindrance of alkoxide group, catalyst |
Figure 1: Reaction pathway of sol-gel processing showing hydrolysis and polycondensation stages with byproduct formation.
Experimental Protocols for Photocatalyst Synthesis
TiOâ‚‚ Photocatalyst via Organic Sol-Gel Route
This protocol produces high-purity TiOâ‚‚ thin films with controlled crystallinity, optimized for photocatalytic applications [4].
Research Reagent Solutions:
Table 2: Essential Reagents for TiOâ‚‚ Photocatalyst Synthesis
| Reagent | Function | Specifications |
|---|---|---|
| Titanium(IV) tetraisopropoxide (TTiP) | Primary titanium precursor | ≥97.0% purity, moisture-sensitive |
| Anhydrous isopropanol (i-PrOH) | Solvent and reaction medium | 99.5%, extra dry over molecular sieves |
| Acetic acid glacial (HAc) | Chelating agent and catalyst | Aldehyde-free |
| Nitric acid 65% (HNO₃) | Catalyst for condensation | Suprapur grade |
| Deionized water (d-H₂O) | Hydrolysis agent | 18.2 MΩ·cm resistivity |
Step-by-Step Procedure:
Solution Preparation: In an inert atmosphere glove box, mix titanium(IV) tetraisopropoxide (TTiP, 0.1 mol) with anhydrous isopropanol (0.4 mol) under vigorous stirring (300 rpm) [4].
Hydrolysis and Chelation: Slowly add acetic acid (0.06 mol per mol TTiP) to control hydrolysis rate. After 30 minutes, add a stoichiometric amount of water (0.4 mol) dropwise to complete hydrolysis. Continue stirring for 3 hours at 25°C until a clear sol forms [4] [5].
Aging and Coating: Age the sol for 24 hours at room temperature. Deposit thin films via dip-coating (withdrawal rate 3 cm/min) or spin-coating (3000 rpm for 30s) onto cleaned substrates [4].
Thermal Treatment: Dry coatings at 80°C for 1 hour, then calcine at 450-500°C for 2 hours (heating rate 5°C/min) to crystallize the anatase phase [4].
Critical Parameters:
- Water-to-precursor ratio: 4:1 (molar)
- pH: ~3-4 (acid-catalyzed)
- Aging time: 24 hours
- Calcination temperature: 450-500°C for anatase formation
ZnO-SiOâ‚‚ Nanocomposite via Aqueous Sol-Gel
This protocol creates homogeneous ZnO-SiOâ‚‚ nanocomposites with interfacial Zn-O-Si bonds, enhancing photocatalytic performance through improved charge separation [6].
Research Reagent Solutions:
Table 3: Essential Reagents for ZnO-SiOâ‚‚ Nanocomposite Synthesis
| Reagent | Function | Specifications |
|---|---|---|
| Tetraethyl orthosilicate (TEOS) | Silicon precursor for SiO₂ matrix | ≥99.0% purity |
| Zinc acetate dihydrate | Zinc oxide precursor | Crystallized ≥99.0% |
| Ethanol absolute | Solvent | Anhydrous |
| Acetic acid | Catalyst for silica network | Analytical grade |
| Sodium hydroxide (NaOH) | Precipitation agent for ZnO | 1 M solution in ethanol |
Step-by-Step Procedure:
Silica Sol Preparation: Prepare a mixture with TEOS:EtOH:H₂O molar ratio of 1:10:4. Add acetic acid (0.05 mol per mol TEOS) as catalyst. Stir vigorously at 300 rpm for 3 hours at 25°C [6].
ZnO Nanoparticle Synthesis: Dissolve zinc acetate dihydrate (10 g in 100 mL ethanol). Gradually add 1 M NaOH solution until precipitation is complete. Age the suspension for 24 hours [6].
Nanocomposite Formation: Mix the zinc hydroxide precipitate with the silica sol in 10:90 (ZnO:SiOâ‚‚) mass ratio. Stir for 2 hours to ensure homogeneous distribution [6].
Gelation and Processing: Transfer the mixture to sealed containers and gel at 75°C for 18 hours. Dry the wet gel at 120°C, then anneal at 450-700°C for 4 hours to form crystalline ZnO within the amorphous SiO₂ matrix [6].
Critical Parameters:
- Ethanol-to-TEOS ratio: 10:1 (prevents premature gelation)
- Gelation temperature: 75°C
- Aging time: 18 hours
- Annealing temperature: 450-700°C (controls ZnO crystallinity)
Figure 2: Experimental workflow for ZnO-SiOâ‚‚ nanocomposite synthesis showing sequential stages from precursor preparation to final thermal processing.
Structural Control and Photocatalytic Performance
The photocatalytic efficiency of sol-gel derived metal oxides is profoundly influenced by the structural parameters controlled during synthesis [7] [8]. Key performance-determining factors include:
Crystalline Phase: TiOâ‚‚ exists primarily as anatase, rutile, or brookite phases. Anatase (band gap = 3.2 eV) demonstrates superior photocatalytic activity compared to rutile (band gap = 3.02 eV) due to its higher Fermi level and slower charge carrier recombination [9] [8].
Surface Area and Porosity: The sol-gel process creates materials with high surface areas (up to 850 m²/g) and controlled pore sizes (typically 20-100 Å), enhancing pollutant adsorption and active site accessibility [1] [4]. The drying method determines final porosity - supercritical drying produces aerogels with >90% porosity, while ambient pressure drying yields xerogels with more moderate surface areas [2].
Dopant Incorporation: Homogeneous distribution of transition metal (Fe, Mn) or noble metal (Ag, Pt) dopants at the molecular level enhances visible light absorption and electron-hole separation [9] [4]. Silver nanoparticles (1-3 wt%) in TiOâ‚‚ act as electron traps, reducing recombination and improving pharmaceutical degradation efficiency by ~40% compared to undoped TiOâ‚‚ [4].
Table 4: Structural Parameters and Their Influence on Photocatalytic Performance
| Structural Parameter | Control Methods | Impact on Photocatalysis |
|---|---|---|
| Crystalline Phase | Calcination temperature, precursors | Anatase TiO₂ (≤500°C) shows highest activity; mixed phases can enhance performance via heterojunctions |
| Specific Surface Area | Water-to-precursor ratio, aging time, drying method | Higher surface area (>100 m²/g) increases active sites and pollutant adsorption capacity |
| Pore Size Distribution | Template agents, catalyst type, solvent | Mesopores (20-500 Ã…) facilitate diffusion of organic pollutants to active sites |
| Dopant Distribution | Precursor chemistry, mixing sequence | Homogeneous doping extends light absorption to visible range and reduces electron-hole recombination |
| Particle Size | Hydrolysis rate, calcination conditions | Smaller nanoparticles (<50 nm) reduce charge carrier migration distance to surface |
Advanced Applications in Photocatalysis
Sol-gel derived metal oxide photocatalysts demonstrate exceptional performance in environmental remediation and energy applications [7] [8]:
Pharmaceutical Degradation: Silver-doped TiOâ‚‚ thin films prepared via organic sol-gel routes show remarkable efficiency in degrading 15 pharmaceutical compounds including antibiotics, endocrine disruptors, and analgesics in wastewater treatment [4]. The modified TiOâ‚‚ with Evonik P25 and Ag nanoparticles demonstrated superior degradation efficiency under UV illumination.
Volatile Organic Compound (VOC) Removal: TiO₂-based photocatalysts effectively mineralize VOCs like formaldehyde, benzene, and toluene in indoor air through photocatalytic oxidation, generating harmless CO₂ and H₂O as final products [9]. The hydroxyl radicals (•OH) generated on illuminated TiO₂ surfaces non-selectively oxidize diverse organic contaminants.
Hydrogen Production: ZnO-SiOâ‚‚ and doped TiOâ‚‚ nanocomposites facilitate photocatalytic water splitting under UV and visible light irradiation. The sol-gel method enables precise control of metal dopants that enhance charge separation and reduce overpotential for hydrogen evolution [7] [6].
The sol-gel process continues to enable sophisticated material architectures for advanced photocatalytic applications, with ongoing research focusing on visible-light activation, heterojunction engineering, and hybrid organic-inorganic systems to address global environmental and energy challenges.
The sol-gel method has emerged as a powerful and versatile synthetic route for the preparation of advanced metal oxide photocatalysts. This wet-chemical technique enables precise control over material architecture at the nanometric scale, offering significant advantages for environmental remediation and energy applications. Within the broader context of sol-gel research for photocatalyst development, three fundamental benefits distinguish this approach: exceptional compositional control, superior homogeneity, and low-temperature processing capabilities. These characteristics are critical for tailoring photocatalytic properties such as band gap energy, surface characteristics, and charge carrier dynamics, ultimately determining efficiency in applications like water treatment and air purification [10] [9]. This document details these advantages through quantitative data, experimental protocols, and material guidelines to support research and development efforts.
Key Advantages and Quantitative Data
The sol-gel process provides distinct, quantifiable benefits for photocatalyst development, as demonstrated by recent research. The table below summarizes key performance metrics and structural properties achievable through sol-gel synthesis.
Table 1: Key Advantages of the Sol-Gel Method for Photocatalyst Synthesis
| Advantage | Key Feature | Experimental Outcome / Quantitative Data | Impact on Photocatalytic Performance |
|---|---|---|---|
| Compositional Control | Precise dopant incorporation [11] | Ni doping in TiOâ‚‚ reduced band gap from 3.11 eV (undoped) to 2.49 eV (0.20 wt.% Ni) [11]. | Enables visible-light absorption; enhances solar efficiency. |
| Homogeneity | Molecular-level mixing [10] | Synthesis of homogeneous, reproducible nanoparticles with easily tuned particle size [10]. | Improves charge carrier mobility; reduces recombination sites. |
| Low-Temperature Processing | Crystallization at ≤350°C [12] | Fabrication of crystalline BiFeO₃ and β-Bi₂O₃ films on flexible polyimide substrates [12]. | Enables use of temperature-sensitive substrates (polymers, textiles). |
Experimental Protocols
General Workflow for Sol-Gel Photocatalyst Synthesis
The following diagram illustrates the generalized workflow for synthesizing metal oxide photocatalysts via the sol-gel method, highlighting stages where key advantages are manifested.
Detailed Protocol: Synthesis of Ni-Doped TiOâ‚‚ (Ni-TiOâ‚‚) for Paracetamol Degradation
This protocol details the synthesis of visible-light-active Ni-TiOâ‚‚ photocatalysts, demonstrating high compositional control and homogeneity for pharmaceutical pollutant removal [11].
Materials
- Titanium precursor: Titanium tetraisopropoxide (TTIP, Câ‚â‚‚H₂₈Oâ‚„Ti ≥ 97%)
- Dopant precursor: Nickel(II) acetate tetrahydrate (Ni(CO₂CH₃)₂·4H₂O ≥ 98%)
- Solvent: Isopropyl alcohol
- Catalyst/Stabilizer: Acetic acid (CH₃COOH, 99.7%)
- Target pollutant: Acetaminophen (Paracetamol, CH₃CONHC₆H₄OH)
Step-by-Step Procedure
- Solution Preparation: Dissolve the required amount of TTIP in half of the total isopropyl alcohol volume under vigorous magnetic stirring (300 rpm) to form Solution A [13].
- Dopant Incorporation: Dissolve nickel(II) acetate tetrahydrate in a mixture of the remaining isopropyl alcohol and acetic acid. Acetic acid acts as a catalyst and chelating agent to stabilize the precursor and control hydrolysis rates [11] [13].
- Mixing and Hydrolysis: Slowly add the nickel-containing solution (Solution B) dropwise to Solution A under continuous stirring. This step initiates the hydrolysis reaction:
M–OR + H₂O → M–OH + ROH[14]. - Condensation and Gelation: Continue stirring the mixture for 6-12 hours at room temperature until a stable, colloidal sol forms. Condensation reactions proceed as:
M–OH + HO–M → M–O–M + H₂OandM–OH + RO–M → M–O–M + ROH(where M = Ti or Ni), building the metal-oxygen network [10] [14]. - Aging and Drying: Age the gel for 24 hours at room temperature, then transfer to a drying oven at 100°C for 24 hours to remove the solvent and obtain a xerogel.
- Calcination: Calcine the dried xerogel in a muffle furnace at 400-500°C for 1-4 hours to crystallize the amorphous material into the desired anatase phase of Ni-TiO₂ and remove residual organics [11].
Photocatalytic Testing Protocol
- Reactor Setup: Use a slurry photoreactor equipped with a visible light source (λ ≥ 420 nm).
- Reaction Conditions: Suspend the Ni-TiOâ‚‚ catalyst (3 g Lâ»Â¹) in an aqueous solution of paracetamol (5 ppm). Maintain constant air bubbling to provide oxygen.
- Activity Assessment: Monitor degradation by measuring the reduction in Total Organic Carbon (TOC). Under optimal conditions, Ni(0.1%)-TiOâ‚‚ achieves 88% TOC removal after 180 minutes [11].
- Stability Testing: Recover the catalyst by centrifugation and reuse for multiple cycles. High-performance catalysts retain ~84% efficiency after five cycles [11].
The Scientist's Toolkit: Essential Research Reagents
The following table lists key reagents and their critical functions in sol-gel synthesis of metal oxide photocatalysts.
Table 2: Essential Research Reagents for Sol-Gel Photocatalyst Synthesis
| Reagent Category | Specific Examples | Primary Function in Synthesis |
|---|---|---|
| Metal Precursors | Titanium tetraisopropoxide (TTIP), Metal acetates (e.g., Calcium, Cobalt, Europium acetate) [11] [14] | Source of metal oxide network; determines stoichiometry and phase purity. |
| Dopant Sources | Nickel(II) acetate tetrahydrate [11], Europium(III) acetate hydrate [14] | Introduces impurity levels to modify band gap; enhances visible-light absorption. |
| Solvents | Isopropyl Alcohol, Ethanol [13] [15] | Dissolves precursors; controls reaction medium viscosity. |
| Catalysts/Stabilizers | Acetic Acid, Hydrochloric Acid (HCl) [13] | Catalyzes hydrolysis/condensation; chelates metals to control reaction kinetics and prevent precipitation. |
| Structure-Directing Agents | Poly(vinyl alcohol) - PVA [14] | Polymer precursor that aids complexation and modifies final material morphology and porosity. |
| 1-Ethoxyheptane-1-peroxol | 1-Ethoxyheptane-1-peroxol | 1-Ethoxyheptane-1-peroxol is a specialty organic peroxide for research (RUO) as a radical initiator or oxidant. It is for laboratory use only and not for human consumption. |
| Stannane, butyltriiodo- | Stannane, butyltriiodo-, CAS:21941-99-1, MF:C4H9I3Sn, MW:556.54 g/mol | Chemical Reagent |
Advanced Low-Temperature Processing Strategies
Conventional calcination remains a critical step for crystallization. However, advanced strategies can further reduce thermal budgets, enabling direct integration with flexible substrates.
Table 3: Strategies for Low-Temperature Crystallization of Sol-Gel Photocatalysts
| Strategy | Mechanism | Protocol Summary | Outcome |
|---|---|---|---|
| UV-Light Assistance [12] | Photochemical breaking of chemical bonds and formation of new ones; creates a high-densified amorphous network that crystallizes more easily. | Synthesize photosensitive precursor solutions (e.g., with β-diketonate complexes). Irradiate deposited layers with continuous UV-excimer lamps during processing. | Crystallization of ferroelectric BiFeO₃ and β-Bi₂O₃ films at temperatures ≤ 350°C on polyimide. |
| Photosensitive Precursors [12] | Utilizes Metal-to-Ligand Charge Transfer (MLCT) transitions in metal complexes under UV light to advance organic removal and network formation. | Use metal complexes (e.g., with N-methyldiethanolamine) with high UV absorption. Irradiate the solution-deposited layer. | Formation of crystalline β-Bi₂O₃ at 250°C. |
| Heterogeneous Photocatalysis of Precursors [12] | TiO₂ particles in the precursor solution act as internal photocatalysts, breaking down organic entities upon UV irradiation before film deposition. | Add TiO₂ particles to the precursor solution. Irradiate the suspension, then remove particles via centrifugation. Use the "pre-catalyzed" solution for deposition. | Low-temperature fabrication of crystalline BiFeO₃ films. |
Application Notes: Performance of Sol-Gel Synthesized Metal Oxide Photocatalysts
The performance of metal oxide photocatalysts is critically dependent on their band gap energy and charge carrier dynamics, which can be precisely tuned through sol-gel synthesis parameters and compositional engineering.
Quantitative Analysis of Band Gap Engineering and Photocatalytic Efficiency
Table 1: Band Gap Engineering and Photocatalytic Performance of Sol-Gel Derived Metal Oxides
| Photocatalyst Material | Synthesis Details | Band Gap (eV) | Surface Area (m²/g) | Photocatalytic Performance | Reference |
|---|---|---|---|---|---|
| In-doped TiO₂ (0.25 wt%) | Sol-gel reflux method | 3.31 → 2.97 (tunable with In concentration) | 102.998 | 85% MB degradation in 8 h (UV) | [16] |
| ZnO (Ethanol solvent) | Modified sol-gel | Not specified | Not specified | 98% MB degradation in "brief duration" | [17] |
| 90TiO₂-10Fe₂O₃/PVP | Sol-gel method | Reduced vs. pure TiO₂ (exact value not specified) | Not specified | Enhanced TCH degradation under UV/solar light | [18] |
| BaTiâ‚…Oâ‚â‚ (PEG-200) | Sol-gel method | 3.61 | 9.78 | Complete MB degradation in 30 min (UV) | [19] |
| WO₃ (Reference) | Various methods | 2.6-2.8 | Not specified | Theoretical STH efficiency up to 4.8% | [20] |
Charge Carrier Dynamics Fundamentals
The efficiency of photocatalytic reactions is governed by charge carrier dynamics, which include:
- Charge Generation: Electron-hole pair creation upon photon absorption with energy exceeding the band gap [20]
- Charge Separation: Prevention of recombination through doping, heterojunctions, or morphology control [21]
- Charge Migration: Movement of carriers to surface reaction sites [22]
- Charge Utilization: Participation in surface redox reactions [7]
The slow kinetics of photogenerated holes and fast recombination of charge carriers represent significant challenges in metal oxide photocatalysts such as WO₃ [20]. Research indicates that transition metal oxides (TiO₂, ZnO, NiO) face fundamental questions regarding the nature of elementary electronic excitations and how these excitations evolve after being created [22].
Experimental Protocols
Protocol: Sol-Gel Synthesis of Indium-Doped TiOâ‚‚ Nanoparticles for Enhanced Photocatalysis
Objective: To synthesize indium-doped TiOâ‚‚ nanoparticles with controlled band gap and improved charge carrier separation for photocatalytic degradation of organic pollutants.
Materials and Equipment
Table 2: Essential Research Reagents and Equipment
| Category | Item | Specification | Function/Purpose |
|---|---|---|---|
| Precursors | Titanium tetraisopropoxide | 97% purity | Primary TiOâ‚‚ source |
| Indium(III) salt | Varying concentrations (0.25-0.75 wt%) | Dopant for band gap engineering | |
| Solvents | Absolute ethanol | Anhydrous | Solvent for hydrolysis |
| 1-Propanol | Laboratory grade | Alternative solvent for comparison | |
| 1,4-Butanediol | Laboratory grade | High-boiling point solvent | |
| Equipment | Reflux apparatus | Standard setup | Controlled hydrolysis & condensation |
| Muffle furnace | Up to 600°C | Calcination and crystallization | |
| Magnetic stirrer | With heating capability | Solution mixing and gel formation |
Step-by-Step Procedure
Solution Preparation
- Dissolve titanium tetraisopropoxide (TTIP) in 50 mL of selected solvent (ethanol, 1-propanol, or 1,4-butanediol) with magnetic stirring at 50°C for 30 minutes [17]
- Prepare indium dopant solution by dissolving appropriate mass of indium precursor in 25 mL of the same solvent to achieve target doping concentration (0.25, 0.50, or 0.75 wt%) [16]
Mixing and Gelation
- Slowly add indium solution to TTIP solution with continuous stirring at 70°C
- Continue stirring until viscous gel forms (typically 1-2 hours)
- Age the gel overnight at 80°C to complete hydrolysis and condensation reactions
Calcination and Crystallization
Characterization
Protocol: Photocatalytic Activity Assessment via Methylene Blue Degradation
Objective: To quantitatively evaluate photocatalytic performance of synthesized metal oxides through degradation of methylene blue (MB) under UV irradiation.
Materials and Setup
- Photocatalyst powder (100 mg)
- Methylene blue solution (5 mg/L, 100 mL) [17]
- UV light source (e.g., Philips TL 8W BLB lamps, λ = 365 nm) [17] [18]
- Magnetic stirrer with illumination chamber
- UV-Vis spectrophotometer for concentration monitoring
Experimental Procedure
Reaction Setup
Kinetic Monitoring
- Collect 3 mL aliquots at regular time intervals (0, 15, 30, 60, 120, 240, 480 minutes)
- Centrifuge aliquots to remove catalyst particles
- Measure absorbance of supernatant at λmax = 664 nm using UV-Vis spectrophotometer
- Calculate degradation percentage using formula: Degradation % = [(A₀ - Aₜ)/A₀] × 100 where A₀ = initial absorbance, Aₜ = absorbance at time t
Performance Validation
- Compare degradation kinetics with undoped TiOâ‚‚ reference
- Calculate apparent rate constant (k) from linear regression of ln(A₀/Aₜ) vs. time
- For optimal 0.25 wt% In-doped TiOâ‚‚, expect ~85% degradation after 8 hours UV irradiation [16]
Advanced Tuning Strategies for Enhanced Performance
Band Gap Engineering Approaches
Table 3: Band Gap Engineering Strategies for Metal Oxide Photocatalysts
| Strategy | Mechanism | Effect on Band Gap | Example System | Performance Outcome |
|---|---|---|---|---|
| Cation Doping | Incorporation of foreign cations into crystal lattice | Gradual decrease with dopant concentration | In-doped TiOâ‚‚ [16] | 85% MB degradation (8 h UV) |
| Composite Formation | Heterojunction interface engineering | Effective band gap narrowing through interface states | TiO₂-Fe₂O₃/PVP [18] | Enhanced tetracycline degradation |
| Solvent Selection | Control of nucleation vs. growth kinetics | Indirect effect through quantum confinement | BaTiâ‚…Oâ‚â‚ with PEG-200 [19] | Complete MB degradation (30 min UV) |
| Morphology Control | Surface area and active site optimization | Minor direct effect, major impact on carrier transport | ZnO with ethanol solvent [17] | 98% MB degradation in brief duration |
Charge Carrier Separation Enhancement
Advanced heterostructure design represents a powerful approach to improve charge carrier separation:
- Type-II Heterojunctions: Create spatial separation of electrons and holes across material interfaces [21]
- Z-Scheme Systems: Mimic natural photosynthesis for simultaneous high redox power and charge separation [23]
- Morphology Control: Low-dimensional nanostructures (2D sheets, 1D rods) shorten carrier migration paths to surfaces [23]
The construction of metal oxide-based composites with complementary materials (carbon-based structures, polymers, other semiconductors) creates synergistic effects that enhance photocatalytic activity through improved charge separation, broadened light absorption, and increased surface area [21].
Troubleshooting and Optimization Guidelines
Common Synthesis Challenges and Solutions
Problem: Rapid hydrolysis and precipitation Solution: Use higher boiling point solvents (e.g., 1,4-butanediol instead of ethanol) to slow reaction kinetics [17]
Problem: Poor crystallinity after calcination Solution: Optimize calcination temperature (500-600°C typically optimal for anatase phase) and duration [16] [18]
Problem: Agglomeration of nanoparticles Solution: Introduce capping agents (e.g., PVP) during synthesis to control particle growth and dispersion [18]
Performance Optimization Parameters
- Dopant Concentration: Optimize around 0.25-0.75 wt% for metal dopants; excessive doping creates recombination centers [16]
- Solvent Selection: Higher viscosity solvents (PEG-200) promote nucleation over growth, yielding higher surface areas [19]
- Calcination Temperature: Balance between crystallinity (higher T) and surface area (lower T) for optimal activity [18]
These application notes and protocols provide a comprehensive framework for designing, synthesizing, and evaluating advanced metal oxide photocatalysts with tailored band structures and enhanced charge carrier dynamics for superior photocatalytic performance.
Metal oxide semiconductors, particularly titanium dioxide (TiOâ‚‚), zinc oxide (ZnO), and silicon dioxide (SiOâ‚‚), represent a cornerstone of modern photocatalytic research for environmental and energy applications. Their efficacy stems from unique physicochemical properties, including high surface area, tunable band gaps, and exceptional thermal and chemical stability [24]. Among various synthesis techniques, the sol-gel method stands out for fabricating these nanomaterials and their complex mixed-oxide composites. This chemical solution process allows for precise atomic-level control over composition and structure, facilitating the creation of tailored materials with enhanced photocatalytic performance, improved charge separation, and extended functional lifetime [25] [26]. This document provides a detailed overview of these metal oxide systems, their synergistic effects in mixed configurations, and standardized protocols for their synthesis and evaluation, framed within the context of advanced sol-gel research.
Individual Metal Oxide Systems: Properties and Functions
Titanium Dioxide (TiOâ‚‚)
TiOâ‚‚ is one of the most widely investigated photocatalysts due to its strong oxidative power, chemical inertness, non-toxicity, and relative cost-effectiveness [27] [25]. It primarily exists in two crystalline phases relevant to photocatalysis: anatase (band gap ~3.2 eV) and rutile (band gap ~3.0 eV). Anatase is generally recognized for its superior photocatalytic activity [28]. A key limitation of pure TiOâ‚‚ is its large band gap, which restricts its photoactivation to ultraviolet (UV) light, and the rapid recombination of its photogenerated electron-hole pairs [29]. The sol-gel method enables the production of TiOâ‚‚ nanoparticles with high surface area and controlled crystallinity, making it indispensable for applications ranging from water splitting to pollutant degradation [25] [26].
Zinc Oxide (ZnO)
ZnO is an n-type semiconductor with a wide direct band gap of ~3.37 eV [30]. It offers high electron mobility, which is beneficial for transporting photogenerated charge carriers and can lead to higher photocatalytic efficiencies in certain reactions compared to TiOâ‚‚ [29]. Beyond photocatalysis, ZnO exhibits potent antibacterial activity, making it suitable for biomedical coatings and self-cleaning surfaces [30] [31]. Similar to TiOâ‚‚, its wide band gap confines its activity to UV light, and it can suffer from photocorrosion. Sol-gel synthesis allows for the creation of diverse ZnO nanostructures, from nanoparticles to nanorods, by simply varying parameters like precursor concentration and pH [30].
Silicon Dioxide (SiOâ‚‚)
SiO₂ is primarily known as an electrical insulator and is not an active photocatalyst itself. However, its role in composite systems is crucial. SiO₂ serves as an excellent support matrix or host for active metal oxides like TiO₂ and ZnO due to its high surface area, thermal stability, and chemical inertness [30] [32]. When integrated into a composite, SiO₂ inhibits the agglomeration of photocatalytic nanoparticles, enhances the material's adsorptive capacity, and improves the dispersion and adhesion of the active phase in coatings [30] [26]. Furthermore, the formation of chemical bonds (e.g., Ti–O–Si) at the interface can stabilize the composite and modify the electronic properties of the active photocatalyst [30] [32].
Mixed Metal Oxide Systems: Synergistic Enhancements
Combining individual metal oxides creates mixed oxide systems where synergistic effects lead to performance metrics surpassing those of the individual components. Key enhancements include increased surface area, suppressed electron-hole recombination, and extended light absorption into the visible range.
Table 1: Photocatalytic Performance of Selected Mixed Oxide Systems
| Composite System | Optimal Composition | Target Pollutant/Reaction | Reported Efficiency | Key Enhancement Mechanism |
|---|---|---|---|---|
| TiO₂–SiO₂ [33] [32] | 85:15 (Ti:Si molar ratio) | Rhodamine B (RhB) dye | ~92% degradation in 6 h [33] | Increased surface area; formation of Ti–O–Si bonds; better nanoparticle dispersion. |
| W-TiOâ‚‚ [27] | 2 mol% W | Methylene Blue (MB) | Peak photocatalytic decomposition | Enhanced charge separation; visible light activation. |
| W-TiOâ‚‚ [27] | 15 mol% W | Methylene Blue (MB) | Most effective overall removal (adsorption + photocatalysis) | Maximal adsorption capacity synergy. |
| Ti–Fe Mixed Oxide [25] | 5 wt.% Fe | Hydrogen generation from water | Higher activity than pure TiO₂ | Fe³⺠acts as electron-hole sink, improving charge separation. |
| ZnO–SiO₂ [30] | 10:90 (Zn:Si) | (Material designed for H₂ sorption) | Increased specific surface area | SiO₂ matrix prevents ZnO aggregation. |
| TiOâ‚‚/ZnO/SiOâ‚‚ [29] | Nanocomposite | Removal of Pb(II) ions | Effective under visible light | Combined benefits of all three oxides; narrower band gap. |
Charge Separation and Visible Light Activation
The interface between two metal oxides can create a energy gradient that drives the separation of photogenerated electrons and holes. For instance, in W-TiOâ‚‚ systems, Wâ¶âº states can act as electron traps, reducing the recombination rate of charge carriers and thus enhancing photocatalytic efficiency [27]. Similarly, doping TiOâ‚‚ with Fe³⺠introduces new energy levels within the band gap, enabling the material to absorb photons from the visible portion of the solar spectrum [25]. This is a critical advancement for deploying photocatalysts under natural sunlight.
Experimental Protocols: Sol-Gel Synthesis and Characterization
This section provides detailed methodologies for the synthesis and evaluation of mixed oxide photocatalysts, based on published research.
Protocol 1: Synthesis of TiO₂–SiO₂ Nanocomposite
This protocol is adapted from the synthesis of highly active TiO₂–SiO₂ nanocomposites for dye degradation [33] [32].
Research Reagent Solutions: Table 2: Essential Reagents for TiO₂–SiO₂ Nanocomposite Synthesis
| Reagent | Function in Synthesis |
|---|---|
| Titanium(IV) Isopropoxide (TTIP) | Precursor for TiOâ‚‚ nanoparticles. |
| Tetraethyl Orthosilicate (TEOS) | Precursor for the SiOâ‚‚ matrix. |
| Ethanol (Câ‚‚Hâ‚…OH) | Solvent; provides homogenization between precursors and water. |
| Hydrochloric Acid (HCl, 0.5 M) | Acid catalyst for hydrolysis and condensation reactions. |
| Deionized Water | Reactant for hydrolysis of metal alkoxides. |
Step-by-Step Procedure:
- Preparation of SiOâ‚‚ Sol: Add 1 mol of TEOS to a solution of 30 mol of ethanol. Under constant stirring, add 3 mL of 0.5 M HCl dropwise. Continue stirring for 3 hours at room temperature [32].
- Preparation of TiOâ‚‚ Sol: In a separate vessel, add 1 mol of TTIP to a solution of 30 mol of isopropanol. Under constant stirring, add 3 mL of 0.5 M HCl dropwise. Continue stirring for 3 hours at room temperature [32].
- Mixing and Gelation: Combine the SiOâ‚‚ and TiOâ‚‚ sols in a 1:1 molar ratio (or as required for the target composition, e.g., 85:15 for TiOâ‚‚:SiOâ‚‚). Stir the mixture for 45 minutes at room temperature to form a homogeneous mixed oxide gel [33] [32].
- Aging and Drying: Allow the gel to age and dry naturally at room temperature until a solid "dry gel" is obtained. Crush this gel into a fine powder.
- Calcination: Subject the powder to calcination in a furnace at 600°C for 5 hours. This step removes residual organic solvents, stabilizes the material, and develops the crystalline anatase phase of TiO₂ [32].
Protocol 2: Synthesis of W-TiOâ‚‚ Nanopowder
This protocol outlines the synthesis of W-TiOâ‚‚ nanopowders with enhanced adsorption and photocatalytic properties [27].
Step-by-Step Procedure:
- Solution A (TTIP Solution): Dissolve Titanium Isopropoxide (TTIP) in 2-propanol under stirring at 500 rpm for 15 minutes to achieve a total precursor concentration (CTi + CW) of 0.3 mol/L.
- Solution B (Water/Alcohol): Mix the required amount of deionized water with 2-propanol separately, with a hydrolysis ratio of h = CH2O/(CTi + CW) = 1.25. Stir for 15 minutes.
- Hydrolysis and Nanoparticle Nucleation: Slowly add Solution B into Solution A under intense stirring. This triggers the hydrolysis of TTIP and the nucleation of titanium oxo-alkoxy nanoparticles.
- Doping with Tungsten: To incorporate tungsten, dissolve the required amount of Tungsten(VI) Chloride (WCl₆) in 2-propanol to create the doping solution. This can be added during the mixing stage to achieve the desired molar ratio (x = CW/(CW + CTi)).
- Drying and Calcination: Allow the colloidal solution to dry in a fume hood at room temperature for 5 days. Subsequently, dry the resulting amorphous powder in an oven at 85°C for 2 days. Finally, calcine the powder in a furnace at 450–600°C for 4 hours to crystallize the anatase phase.
Key Characterization Techniques
- X-ray Diffraction (XRD): Used to identify crystalline phases, estimate crystallite size using the Scherrer equation, and determine phase composition (e.g., anatase vs. rutile in TiOâ‚‚) [30] [32].
- UV-Vis Spectroscopy: Employed to study the electronic excitation behavior of the material and estimate the band gap energy via Tauc plots [33] [31].
- Scanning Electron Microscopy (SEM) & Energy-Dispersive X-ray Spectroscopy (EDX): Provide insights into surface morphology, particle size, and elemental composition/homogeneity [33] [30].
- Fourier-Transform Infrared (FT-IR) Spectroscopy: Identifies functional groups and confirms the formation of chemical bonds in composites, such as Ti–O–Si or Zn–O–Si [33] [30].
- Nitrogen Physisorption (BET): Measures the specific surface area, pore volume, and pore size distribution, which are critical for adsorption and catalytic activity [25].
Visualizing Synthesis and Charge Separation Pathways
The following diagrams illustrate the core workflows and mechanisms involved in mixed oxide photocatalysis.
Diagram 1: Generalized sol-gel synthesis workflow for mixed oxides.
Diagram 2: Enhanced charge separation mechanism in a W-TiOâ‚‚ mixed oxide.
The sol-gel method is a versatile and powerful chemical technique for synthesizing metal oxide photocatalysts, offering unparalleled control over material properties at the nanoscale. This bottom-up approach involves the transition of a solution system from a liquid "sol" into a solid "gel" phase, enabling precise manipulation of the final material's morphology, porosity, and surface characteristics [2] [34]. In photocatalytic applications, where performance is critically dependent on surface area, charge transport, and light absorption, the ability to engineer nanostructure through sol-gel chemistry provides a fundamental advantage [7] [21]. By carefully controlling synthesis parameters, researchers can tailor photocatalytic materials with enhanced activity for applications ranging from environmental remediation to energy conversion [35].
The connection between synthetic control and functional performance is paramount. Photocatalytic efficiency depends on a material's capacity to absorb light, generate charge carriers (electrons and holes), and facilitate their migration to the surface to drive chemical reactions without recombination [7]. Nanostructuring directly influences each of these processes; for instance, high surface area provides more active sites, controlled porosity affects molecular diffusion, and crystalline phase impacts electronic band structure [36] [37]. The sol-gel process, through its molecular-level mixing and low-temperature processing, allows for precise tuning of these structural parameters to overcome common limitations in photocatalysis, such as rapid electron-hole recombination and limited visible-light absorption [3] [21].
Key Parameters for Morphology Control in Sol-Gel Synthesis
The sol-gel technique provides multiple adjustable parameters that directly influence the morphology and, consequently, the photocatalytic performance of the resulting metal oxides. Understanding and controlling these parameters is essential for designing efficient photocatalysts.
Table 1: Key Sol-Gel Synthesis Parameters and Their Impact on Final Material Properties
| Control Parameter | Impact on Morphology & Structure | Effect on Photocatalytic Activity |
|---|---|---|
| Precursor Type | Determines crystallite size, phase formation temperature, and grain morphology [38]. | Influences light absorption edge, charge carrier mobility, and surface defect chemistry [38] [34]. |
| Surfactant/Template | Directly controls porosity, specific surface area (BET), and particle shape (e.g., spherical, fibrous) [36]. | Higher surface area increases active sites; pore size affects reactant diffusion; morphology can enhance light harvesting [36]. |
| Catalyst (pH) | Affects the gel network structure (polymeric vs. particulate) and its density [2] [34]. | Alters the density of surface hydroxyl groups and the rate of electron-hole recombination [34]. |
| Annealing Temperature | Controls crystallinity, crystallite size, phase composition, and defect concentration (e.g., oxygen vacancies) [38] [37]. | Improved crystallinity typically reduces charge recombination; excessive temperature can reduce surface area [37]. |
| Sol Aging Duration | Influences the viscosity of the sol and the thickness and porosity of deposited films [35]. | Optimized aging leads to higher porosity and better accessibility to active sites, enhancing performance [35]. |
The relationship between these parameters and the resulting photocatalytic activity is often synergistic. For example, research on ZrO₂ thin films demonstrated that using zirconium acetate as a precursor, combined with an annealing temperature of 500°C and a film thickness of 940 nm, produced a material with superior optical and crystalline quality. This optimally structured film achieved a 94% photocatalytic degradation efficiency of methylene blue dye within 150 minutes, significantly outperforming films made from other zirconium sources like nitrate or chloride [38]. This highlights how the careful selection of a single precursor can dictate the structural and functional outcome.
Furthermore, the use of surfactants and templates represents a powerful strategy for morphological engineering. A study on TiO₂ anatase phase synthesis revealed that using sodium dodecyl sulfate (SDS) as an ionic surfactant resulted in a spherical morphology with a high specific surface area of 138.72 m²/g and a mesoporous structure. These morphological traits improved light absorption in the visible region and gave the material the highest photocatalytic activity for methylene blue degradation compared to samples prepared with other surfactants or no surfactant [36]. The ability to fine-tune such textural properties is a distinct advantage of the sol-gel method.
Experimental Protocols for Morphology-Controlled Synthesis
Protocol: Surfactant-Assisted Sol-Gel Synthesis of Mesoporous TiOâ‚‚
This protocol details the synthesis of high-surface-area, mesoporous TiOâ‚‚ using ionic and non-ionic surfactants, based on the methodology that demonstrated superior photocatalytic performance [36].
Research Reagent Solutions:
- Titanium Alkoxide Precursor: Titanium isopropoxide (Ti(OCH(CH₃)₂)₄) or titanium butoxide (Ti(OC₄H₉)₄). Functions as the primary source of titanium ions.
- Anhydrous Ethanol: Serves as the solvent for the alkoxide precursor.
- Surfactant Solutions:
- SDS (Anionic): 0.1 M Sodium Dodecyl Sulfate in ethanol/water.
- CTAB (Cationic): 0.1 M Cetyltrimethylammonium Bromide in ethanol/water.
- PEG (Non-ionic): 5% w/v Polyethylene Glycol (MW ~10,000) in ethanol/water.
- Acid Catalyst: Dilute nitric acid (HNO₃) or acetic acid (CH₃COOH) to catalyze hydrolysis and condensation.
- Deionized Water: For hydrolysis of the metal alkoxide.
Step-by-Step Procedure:
- Sol Preparation: In a dry beaker, dissolve 10 mmol of the titanium alkoxide in 50 mL of anhydrous ethanol under constant stirring. This forms the precursor solution.
- Surfactant Addition: To this solution, add 50 mL of the chosen surfactant solution (SDS, CTAB, or PEG). Stir the mixture for 1 hour at room temperature to ensure homogeneity.
- Catalyzed Hydrolysis: Slowly add a mixture of 5 mL of the acid catalyst and 5 mL of deionized water dropwise to the stirring solution. The molar ratio of water to alkoxide is critical and should typically be between 4:1 and 10:1.
- Gelation and Aging: Continue stirring for 2-4 hours until the solution becomes translucent and viscous, indicating gel formation. Cover the beaker with perforated parafilm and allow the gel to age at room temperature for 24-48 hours.
- Drying: Transfer the aged gel to an oven and dry at 80°C for 12 hours to remove the solvent, resulting in a xerogel.
- Calcination: Place the xerogel in a furnace and heat to 450-500°C at a ramp rate of 2°C/min. Maintain this temperature for 4 hours to remove the surfactant template completely, crystallize the TiO₂ into the anatase phase, and develop the desired mesoporous structure.
Protocol: Precursor-Dependent Synthesis of ZrOâ‚‚ Thin Films
This protocol outlines the formation of ZrOâ‚‚ thin films with tunable properties using different zirconium sources, a key factor in optimizing photocatalytic activity [38].
Research Reagent Solutions:
- Zirconium Precursors:
- Zirconium(IV) Acetate: Leads to films with high optical and crystalline quality.
- Zirconium(IV) Oxynitrate: For comparison of nitrate-derived properties.
- Zirconium(IV) Oxychloride: For comparison of chloride-derived properties.
- Solvent: A mixture of ethanol and deionized water.
- Complexing Agent: Acetylacetone, which can be added to modify precursor reactivity and stabilize the sol.
Step-by-Step Procedure:
- Sol Formulation: Dissolve 0.1 mol of the selected zirconium precursor in 100 mL of a 1:1 v/v ethanol/water solvent mixture. Stir vigorously until a clear solution is obtained.
- Sol Aging: Allow the sol to age for 24 hours at room temperature. This aging process allows for the initial development of the metal-oxo polymer network, which influences the final film's homogeneity and thickness [35].
- Substrate Preparation: Clean glass substrates sequentially in an ultrasonic bath with acetone, ethanol, and deionized water. Dry the substrates in a stream of nitrogen gas.
- Dip-Coating: Immerse the clean substrate into the aged sol and withdraw it at a controlled, constant rate (e.g., 2-5 cm/min). The withdrawal speed is a primary factor in determining the final film thickness.
- Drying and Pre-annealing: Immediately after deposition, dry the wet film at 100°C for 10 minutes on a hotplate to evaporate the solvent.
- Thermal Annealing: Transfer the film to a pre-heated furnace and anneal at 500°C for 1 hour. This step is crucial for developing the tetragonal crystalline phase of ZrO₂ and removing any residual organics, thereby establishing the final optical and photocatalytic properties [38].
Visualizing the Sol-Gel Morphology Control Pathway
The following workflow diagram illustrates the critical decision points in a sol-gel synthesis and how specific parameter choices lead to distinct material morphologies and photocatalytic outcomes.
The Scientist's Toolkit: Essential Reagents for Sol-Gel Synthesis
Table 2: Key Research Reagent Solutions for Sol-Gel Synthesis of Photocatalysts
| Reagent Category | Specific Examples | Primary Function in Synthesis |
|---|---|---|
| Metal Precursors | Titanium isopropoxide, Zinc acetate, Zirconium(IV) acetate [38] [37] | Source of metal cations; the ligand type (alkoxide, acetate, chloride) governs reactivity, hydrolysis rate, and final grain morphology. |
| Solvents | Ethanol, Isopropanol, Diethylene glycol [37] | Dissolve precursors to form a homogeneous sol; the polarity and boiling point influence reaction kinetics and drying behavior. |
| Surfactants / Templates | SDS, CTAB, PEG, Pluronic polymers [36] | Structure-directing agents that self-assemble to create controlled pore sizes and high surface area, preventing particle agglomeration. |
| Catalysts | Nitric acid, Acetic acid, Ammonium hydroxide [2] [34] | Modify pH to control the relative rates of hydrolysis and condensation, determining gel network structure (polymeric or particulate). |
| Complexing Agents | Acetylacetone, Citric acid [34] | Chelate metal ions to moderate their reactivity, prevent precipitation, and promote atomic-scale homogeneity in multi-metal systems. |
| Urea, (p-hydroxyphenethyl)- | Urea, (p-hydroxyphenethyl)- | Urea, (p-hydroxyphenethyl)- is a chemical for research (RUO). It is not for human, veterinary, or household use. Explore its value as a urease inhibitor and antimicrobial agent. |
| Chloro(diethoxy)borane | Chloro(diethoxy)borane, CAS:20905-32-2, MF:C4H10BClO2, MW:136.39 g/mol | Chemical Reagent |
The sol-gel method stands as a profoundly effective technique for engineering the morphology of metal oxide photocatalysts. By manipulating a suite of interconnected parameters—including precursor chemistry, surfactant templating, catalysis conditions, and thermal treatment—researchers can exert precise control over critical structural properties such as specific surface area, porosity, crystallinity, and defect concentration. This tunability directly translates to enhanced photocatalytic performance by optimizing light absorption, charge carrier separation, and surface reaction kinetics. The provided protocols and data offer a foundational toolkit for designing sol-gel synthesized materials with tailored nanostructures for advanced photocatalytic applications, from environmental remediation to sustainable energy conversion.
Practical Synthesis Protocols and Advanced Material Architectures for Enhanced Photocatalysis
The sol-gel process is a versatile, solution-based chemical method for synthesizing metal oxide nanostructures at relatively low temperatures. This technique is particularly valuable in the field of photocatalysis, as it enables precise control over the composition, morphology, and textural properties of metal oxide materials, which directly influence their light absorption capacity, charge carrier separation, and surface reactivity [21] [5]. The process involves the transformation of a colloidal solution (sol) of precursor molecules into an integrated, three-dimensional network (gel) through a series of hydrolysis and condensation reactions [39] [5]. For photocatalytic applications, such as pollutant degradation and hydrogen production via water splitting, the sol-gel method facilitates the creation of high-purity, homogeneous, and porous metal oxide frameworks (e.g., TiOâ‚‚, ZnO) with high surface areas, which are critical for enhancing catalytic activity [21] [5]. The ability to dope these oxides with other metals or integrate them into composite structures at the molecular level further allows for the fine-tuning of their electronic band structure, thereby improving their responsiveness to visible light [21] [3].
Foundational Chemistry and Principles
The sol-gel process is governed by two primary classes of chemical reactions: hydrolysis and condensation. The sequence and kinetics of these reactions are critical for determining the structure of the resulting gel network and the final properties of the metal oxide material [5].
- Hydrolysis: This initial step involves the nucleophilic attack of a water molecule on a metal atom (M) in the precursor, leading to the replacement of an alkoxide group (OR) with a hydroxyl group (OH) [5].
M(OR)₄ + H₂O → M(OR)₃(OH) + ROH - Condensation: Following hydrolysis, the partially hydrolyzed species link together through polycondensation. This occurs via two main pathways:
- Oxolation:
M-OH + M-OR → M-O-M + ROH - Olation:
M-OH + M-OH → M-O-M + H₂OThese reactions proceed to form an extensive metal-oxygen-metal (M-O-M) network, characteristic of the final metal oxide [5].
- Oxolation:
The rates of these reactions are profoundly influenced by the pH of the solution, which dictates the nucleophilicity of the attacking species and the electrophilicity of the metal center [39] [5]. As visually summarized in the diagram below, the reaction pathway and resulting gel structure diverge significantly under acidic versus basic conditions.
Experimental Protocols
Precursor Selection and Solution Preparation
The selection of precursors is the first critical step in designing a sol-gel synthesis, as it determines the reactivity, purity, and homogeneity of the final metal oxide. The table below categorizes and compares common precursor types.
Table 1: Common Precursor Types in Sol-Gel Synthesis
| Precursor Type | Examples | Key Characteristics | Impact on Final Material |
|---|---|---|---|
| Metal Alkoxides | Titanium isopropoxide, Tetraethyl orthosilicate (TEOS) [39] | High reactivity, sensitive to moisture, produce high-purity oxides [39] | Excellent stoichiometry control, high homogeneity [5] |
| Metal Inorganic Salts | Bismuth nitrate [Bi(NO₃)₃·5Hâ‚‚O], Aluminum nitrate [40] [39] | Lower cost, less sensitive to handling, may require acid for dissolution [40] | Can introduce anionic impurities (e.g., NO₃â», Clâ»); requires careful calcination [40] |
| Chelated Complexes | Citric acid complexes, Acetylacetonate complexes [40] [5] | Modified reactivity, slower condensation, reduced precipitation [40] | Enhanced molecular-level mixing, finer particle size, prevents premature precipitation [40] [5] |
Protocol: Preparation of a Citrate-Modified Precursor Sol for Mixed Oxide Synthesis (e.g., BiBaO₃) [40]
- Dissolution of Metal Salts:
- In Beaker A, dissolve a stoichiometric amount of bismuth nitrate pentahydrate
[Bi(NO₃)₃·5H₂O]in 20 mL of distilled water. - In Beaker B, disperse a stoichiometric amount of barium carbonate
[BaCO₃]in 40 mL of dilute nitric acid[HNO₃]under continuous stirring until a clear solution of barium nitrate is formed and CO₂ evolution ceases.
- In Beaker A, dissolve a stoichiometric amount of bismuth nitrate pentahydrate
- Mixing and Complexation:
- Combine the solutions from Beakers A and B while stirring thoroughly.
- Heat the mixed solution to approximately 70°C while maintaining continuous stirring for 1 hour to ensure homogeneity.
- Add ethylene glycol and citric acid as complexing and gelling agents. A 1:1 molar ratio of the total metal ions to both citric acid and ethylene glycol is typically used.
- Sol Formation:
- Continue heating and stirring the mixture at 70-80°C for several hours (typically 3-5 hours) until a transparent, viscous sol is formed. The solution should remain clear without any visible precipitation.
Catalyst Use and Reaction Control
The type and concentration of the catalyst are powerful tools for directing the sol-gel process toward a desired material structure, as outlined in the reaction pathway diagram. The choice between acid and base catalysis directly affects the reaction kinetics and the morphology of the growing gel network [39] [5].
Table 2: Effect of Catalyst Type on Sol-Gel Kinetics and Gel Structure
| Parameter | Acid Catalysis (pH < 7) | Base Catalysis (pH > 7) |
|---|---|---|
| Hydrolysis Rate | Slower, more controlled [39] | Faster [39] |
| Condensation Mechanism | Favors olation (M-OH + M-OH) or reactions of protonated species, leading to linear polymers [5] | Favors oxolation (M-OR + M-OH), leading to highly branched clusters [5] |
| Primary Reactive Site | Protonated alkoxide group (M-ORHâº) [5] | Deprotonated hydroxyl group (M-Oâ») [5] |
| Resulting Gel Structure | Low-density polymer gel: Entangled linear chains, leading to finer pores and higher specific surface area [39] [5] | High-density particulate gel: Dense, colloidal aggregates of branched clusters, leading to larger pores and lower surface area [39] [5] |
| Typical Catalysts | HCl, HNO₃, Acetic acid [40] [39] | NH₄OH, NaOH [39] |
Protocol: Acid-Catalyzed Synthesis of Monolithic Silica [41] [39]
- Precursor Preparation: Dilute tetraethyl orthosilicate (TEOS) in ethanol (e.g., 1:4 molar ratio TEOS:EtOH).
- Acidified Water Preparation: Mix a precise amount of distilled water (e.g., 4:1 molar ratio Hâ‚‚O:TEOS) with a mineral acid like HCl to achieve a pH of approximately 2.
- Catalyzed Hydrolysis: Slowly add the acidified water to the TEOS/ethanol solution under vigorous stirring. Maintain the reaction at room temperature for 1 hour to allow for controlled, complete hydrolysis.
- Gelation: After hydrolysis, the solution may be cast into a mold. Gelation under acidic conditions will proceed slowly, forming a transparent, monolithic wet gel over a period of hours to days.
Aging Conditions and Gel Modification
Aging is the process of allowing the newly formed gel to remain in its mother liquor for a specific duration. This step is crucial for strengthening the gel network and controlling its porosity through ongoing chemical reactions and physical processes [39].
- Syneresis: The spontaneous shrinkage of the gel and expulsion of liquid from its pores due to the formation of new chemical bonds, which increases the connectivity and mechanical strength of the network [39].
- Ostwald Ripening: A process where smaller, more soluble particles dissolve and re-deposit onto larger, more stable particles. This reduces the surface energy of the system, leading to a more uniform particle size distribution and thickening of gel necks between particles [39].
- Polycondensation: Aging allows further condensation reactions to occur, which strengthens the M-O-M network throughout the gel [39].
Protocol: Controlled Aging and Drying for High-Surface-Area Xerogels [39]
- Sealing and Aging: After gelation, seal the container to prevent solvent evaporation. Allow the gel to age at room temperature for 24-72 hours. For accelerated aging, the process can be conducted at an elevated temperature (e.g., 50-60°C) for a shorter duration (e.g., 6-12 hours).
- Solvent Exchange (Optional but Recommended): To reduce capillary stresses during drying and minimize cracking, slowly replace the pore liquid (e.g., water/ethanol) with a solvent of lower surface tension, such as acetone or hexane. This is done by immersing the aged gel in the new solvent for 12-24 hours, repeating if necessary.
- Drying: The solvent-filled gel is dried slowly at ambient pressure and temperature (xerogel formation) to produce a porous solid. For applications requiring ultra-high porosity, supercritical drying (producing an aerogel) is employed to avoid pore collapse by eliminating liquid-vapor interfaces [41] [39].
Heat Treatment (Calcination)
Calcination is the final thermal treatment step that converts the porous, amorphous xerogel into a crystalline metal oxide with tailored functional properties [39].
Protocol: Standard Calcination for Crystallization [39]
- Loading: Place the dried xerogel powder or monolith in a high-temperature stable crucible.
- Thermal Ramping: Transfer the crucible to a pre-programmed furnace. Heat the sample at a controlled ramp rate (e.g., 2-5°C per minute) to the target temperature. A slow ramp rate is critical to prevent violent combustion of residual organics and avoid cracking or foaming.
- Dwell Time: Maintain the sample at the target temperature (e.g., 400-800°C, depending on the metal oxide) for 2-6 hours. This ensures complete removal of organic species and residual solvents, and allows for full crystallization and growth of the desired crystal phase.
- Atmosphere Control: Perform calcination in a static air atmosphere for most applications. For metals prone to oxidation reduction states, a controlled (inert or reducing) atmosphere may be used to create oxygen vacancies, which can enhance photocatalytic activity [39].
- Cooling: Allow the sample to cool slowly to room temperature inside the turned-off furnace to minimize thermal stress.
The entire sol-gel workflow, from precursor preparation to final heat treatment, is summarized in the following diagram.
The Scientist's Toolkit: Essential Reagents and Materials
Table 3: Key Research Reagent Solutions for Sol-Gel Synthesis
| Reagent / Material | Function / Role | Example in Protocol |
|---|---|---|
| Metal Alkoxides (e.g., TEOS, Ti(OiPr)â‚„) | Primary network formers; source of metal oxide upon hydrolysis and condensation [39] [5] | Titanium isopropoxide for TiOâ‚‚ photocatalyst synthesis [39] |
| Metal Salts (e.g., Nitrates, Chlorides) | Cost-effective alternative precursors; require calcination to remove anionic impurities [40] [39] | Bismuth nitrate for Bi-containing perovskites [40] |
| Solvents (e.g., Ethanol, 2-Methoxyethanol) | Dissolve precursors; medium for chemical reactions; control concentration and viscosity [42] [39] | Ethanol for diluting TEOS [39]; 2-Methoxyethanol as a dominant solvent for BiFeO₃ [42] |
| Mineral Acids/Bases (e.g., HCl, NH₄OH) | Catalysts that control pH, reaction rates, and the structure of the gel network [39] [5] | HCl for acid-catalyzed silica synthesis; HNO₃ for dissolving barium carbonate [40] [39] |
| Chelating Agents (e.g., Citric Acid, Acetylacetone) | Modify precursor reactivity; promote homogeneity in multi-component systems; prevent precipitation [42] [40] | Citric acid as a chelating agent in BiBaO₃ and BiFeO₃ synthesis [42] [40] |
| Drying Control Chemical Additives (DCCAs) (e.g., Formamide, Glycerol) | Reduce capillary pressure during drying by modifying surface tension, minimizing cracking of monoliths [41] | Used during the aging/drying step to produce crack-free monoliths [41] |
| Structure-Directing Agents (Templates) (e.g., Surfactants, Block Copolymers) | Create ordered mesoporous structures by self-assembly; removed during calcination to leave behind pores [5] | Used to create mesoporous TiOâ‚‚ with high surface area for photocatalysis [5] |
| Cyclotetradecane-1,2-dione | Cyclotetradecane-1,2-dione|C14H24O2|CAS 23427-68-1 | Cyclotetradecane-1,2-dione is a 14-membered macrocyclic dione for chemical and conformational research. For Research Use Only. Not for human use. |
| 3-Methyl-5-phenylbiuret | 3-Methyl-5-phenylbiuret|High-Purity Research Chemical | 3-Methyl-5-phenylbiuret for research applications. This compound is For Research Use Only (RUO), not for diagnostic or personal use. |
Concluding Remarks
The sol-gel method offers a powerful and versatile pathway for synthesizing advanced metal oxide photocatalysts. The precise control over each stage of the process—from the initial selection of precursors and catalysts to the careful management of aging and calcination conditions—enables researchers to engineer materials with specific compositional, structural, and textural properties. By adhering to the detailed protocols and principles outlined in this document, scientists can systematically develop and optimize sol-gel-derived photocatalysts, thereby advancing research in environmental remediation and sustainable energy generation.
The sol-gel process represents a versatile chemical technique for the fabrication of metal oxide networks through the transition of a solution system from a liquid "sol" into a solid "gel" phase. While conventional sol-gel methods provide excellent control over composition and homogeneity, recent advancements integrating hydrothermal and microwave assistance have significantly enhanced the capabilities of this synthesis route. These advanced fabrication techniques enable superior control over crystallinity, particle size, morphology, and photocatalytic performance of metal oxides, addressing key limitations of traditional approaches such as irregular crystallinity and prolonged processing times [43] [19] [44].
Within the context of metal oxide photocatalyst synthesis, these advanced sol-gel methods facilitate the creation of materials with tailored properties essential for efficient photocatalytic activity. The synergy between sol-gel chemistry and hydrothermal or microwave energy input has opened new avenues for designing novel photocatalytic materials with enhanced charge separation, reduced recombination losses, and improved quantum efficiency. This technical note provides detailed protocols and application guidelines for researchers developing advanced metal oxide photocatalysts through these innovative synthesis routes.
Hydrothermal-Assisted Sol-Gel Synthesis
Fundamental Principles and Mechanisms
Hydrothermal-assisted sol-gel synthesis combines the molecular-level mixing advantages of conventional sol-gel processing with the crystallinity enhancement offered by hydrothermal treatment. This hybrid approach subjects the gel precursor to elevated temperatures and pressures in a sealed autoclave, creating conditions that promote enhanced crystallization kinetics and thermodynamic stability of the resulting metal oxides. The hydrothermal environment facilitates the dissolution and recrystallization processes, leading to the formation of highly crystalline phases at significantly lower temperatures compared to conventional calcination routes [45] [46].
The mechanism involves several sequential stages: (1) hydrolysis and condensation of metal precursors to form a colloidal sol, (2) gelation through polycondensation reactions, (3) hydrothermal crystallization under autogenous pressure, and (4) Ostwald ripening for crystal growth and morphology control. The elevated temperature and pressure conditions during hydrothermal treatment enhance the solubility and reactivity of the precursor species, promoting direct crystallization from the amorphous gel network. This process typically yields materials with higher phase purity, better-defined morphologies, and improved thermal stability compared to those obtained through conventional sol-gel routes followed by calcination [19].
Experimental Protocol: BaTiO₃ Nanoparticle Synthesis
Materials and Reagents:
- Barium acetate (C₄H₆BaO₄, ACS reagent, ≥99%)
- Titanium butoxide (Ti[O(CH₂)₃CH₃]₄, reagent grade, 97%)
- Polyvinylpyrrolidone (PVP, MW ≈ 40,000)
- Acetic acid (glacial, ≥99%)
- Ethanol absolute (≥99%)
- Sodium hydroxide (NaOH, reagent grade, ≥98%)
- Deionized water (resistivity ≥18 MΩ·cm)
Procedure:
- Precursor Solution Preparation: Dissolve 0.02 mol barium acetate in 40 mL deionized water with continuous stirring at 60°C. Add acetic acid dropwise until complete dissolution is achieved (final pH ≈ 4).
Titanium Solution Preparation: In a separate vessel, mix 0.02 mol titanium butoxide with 40 mL ethanol under nitrogen atmosphere. Add 2 g PVP as a capping agent and stir until complete dissolution.
Sol Formation: Slowly add the titanium solution to the barium solution with vigorous stirring (600 rpm) over 30 minutes. Maintain the temperature at 60°C and continue stirring for 2 hours to form a stable, transparent sol.
Gelation and Aging: Transfer the sol to a sealed container and age at 80°C for 24 hours to form a wet gel. The gel should exhibit a translucent, homogeneous appearance.
Hydrothermal Treatment: Transfer the gel to a Teflon-lined stainless steel autoclave, filling 70-80% of its capacity. Conduct hydrothermal treatment at 180-220°C for 12-48 hours. The specific temperature and duration depend on the desired crystal size and phase composition [45].
Product Recovery: After natural cooling to room temperature, collect the precipitate by centrifugation at 10,000 rpm for 10 minutes. Wash sequentially with deionized water and ethanol three times each to remove impurities.
Drying: Dry the product at 80°C for 12 hours in a vacuum oven to obtain BaTiO₃ nanoparticles.
Post-annealing (Optional): For enhanced crystallinity, anneal the powder at 500-700°C for 2 hours in a muffle furnace (heating rate: 5°C/min) [45].
Critical Parameters and Optimization
The hydrothermal-assisted sol-gel process is highly dependent on several critical parameters that significantly influence the final material properties:
Temperature Effects: Crystallite size and phase composition show strong temperature dependence. For BaTiO₃ synthesis, temperatures between 180-200°C typically yield particles with mean sizes of 120-180 nm, while higher temperatures (220°C) produce larger crystals (300-600 nm) with a peak distribution at 480 nm [45].
Reaction Time: Optimal crystallization generally occurs between 12-24 hours. Shorter durations may result in incomplete crystallization, while extended periods can promote excessive particle growth and agglomeration.
pH Control: The acidity of the precursor solution significantly affects hydrolysis rates. A slightly acidic medium (pH ≈ 4) typically provides optimal conditions for barium titanate formation, controlling both reaction kinetics and final stoichiometry.
Solvent Selection: Solvent properties including boiling point, viscosity, and polarity directly influence particle morphology and surface area. High boiling point solvents with greater viscosity (e.g., PEG-200) slow evaporation, promote nucleation over crystal growth, and minimize agglomeration, resulting in higher surface area materials [19].
Table 1: Optimization Parameters for Hydrothermal-Assisted Sol-Gel Synthesis
| Parameter | Optimal Range | Influence on Product | Remarks |
|---|---|---|---|
| Temperature | 180-220°C | Determines crystallite size and phase purity | Higher temperature → larger crystals |
| Time | 12-48 hours | Affects crystallinity and particle growth | Beyond 24h → minimal size increase |
| pH | 3-5 (acidic) | Controls hydrolysis and condensation rates | Affects stoichiometry in multi-component systems |
| Solvent Type | PEG, EG, Alcohols | Impacts morphology and surface area | High boiling point solvents reduce agglomeration |
| Filling Degree | 70-80% | Influences autogenous pressure | Critical for safety and reproducibility |
Characterization and Performance Evaluation
Comprehensive characterization of hydrothermally synthesized photocatalysts is essential for correlating synthetic parameters with functional properties:
Structural Analysis: X-ray diffraction (XRD) confirms phase formation and crystallinity. For BaTiO₃, the tetragonal phase is characterized by splitting of (002)/(200) peaks at 2θ ≈ 45°. Rietveld refinement provides quantitative phase analysis and crystal structure information [45].
Morphological Examination: Field emission scanning electron microscopy (FESEM) reveals particle size distribution, morphology, and degree of agglomeration. Transmission electron microscopy (TEM) provides detailed information on crystal structure, lattice fringes, and defects.
Surface Area and Porosity: Nitrogen adsorption-desorption analysis determines specific surface area (BET method), pore size distribution, and total pore volume. BaTiO₃ synthesized via hydrothermal-assisted sol-gel typically exhibits surface areas of 10-50 m²/g, with mesoporous characteristics [19].
Optical Properties: UV-Vis diffuse reflectance spectroscopy determines band gap energy through Tauc plot analysis. BaTiO₃ typically shows a band gap of approximately 3.2-3.4 eV, suitable for UV-light-driven photocatalysis.
Photocatalytic Performance: Methylene blue (MB) degradation under UV light irradiation serves as a standard test for photocatalytic activity. BaTiO₃ synthesized using PEG-200 as solvent demonstrated complete MB degradation within 30 minutes, attributed to its high surface area (9.78 m²/g) and optimal pore structure [19].
Microwave-Assisted Sol-Gel Synthesis
Fundamental Principles and Mechanisms
Microwave-assisted sol-gel synthesis utilizes microwave radiation as an energy source to drive the sol-gel process, offering significantly reduced reaction times, enhanced reaction kinetics, and improved product homogeneity compared to conventional heating methods. The fundamental principle involves the interaction of microwave electromagnetic radiation with polar molecules and charged particles in the reaction mixture, leading to rapid, volumetric heating through dipole rotation and ionic conduction mechanisms [43] [44].
The microwave effect extends beyond mere thermal acceleration, potentially influencing reaction pathways through specific non-thermal effects. The rapid and uniform heating minimizes thermal gradients, leading to more homogeneous nucleation and growth conditions. This results in narrower particle size distributions, controlled morphology, and in some cases, unique metastable phases not easily accessible through conventional heating methods. For photocatalyst synthesis, these characteristics translate to materials with enhanced surface activity and improved charge carrier dynamics [47] [44].
Experimental Protocol: Fe₂O₃@TiO₂ Core-Shell Nanocomposite Synthesis
Materials and Reagents:
- Iron (III) oxide nanoparticles (Fe₂O₃, 12-75 nm)
- Titanium tetra-n-butoxide (Ti(C₄H₉O)₄, reagent grade, ≥97%)
- Ethanol absolute (≥99%)
- Ammonia solution (NHâ‚„OH, 28%)
- Deionized water (resistivity ≥18 MΩ·cm)
Procedure:
- Core Dispersion Preparation: Disperse 0.5 g commercial Fe₂O₃ nanoparticles in 100 mL ethanol using ultrasonic bath treatment for 30 minutes to achieve a homogeneous suspension.
Titanium Precursor Solution: In a separate container, mix 0.1 mol titanium tetra-n-butoxide with 50 mL ethanol under continuous stirring. Add 5 mL deionized water dropwise to initiate partial hydrolysis.
Coating Process: Slowly add the titanium precursor solution to the Fe₂O₃ dispersion with vigorous mechanical stirring (800 rpm). Simultaneously, add ammonia solution (2 mL) to maintain pH ≈ 9, catalyzing the condensation reaction.
Microwave Irradiation: Transfer the reaction mixture to a microwave-compatible reaction vessel. Irradiate using a microwave synthesis system at 300 W, maintaining temperature at 80°C for 20-30 minutes. Use magnetic stirring (500 rpm) throughout the irradiation process to ensure uniform heating [43].
Product Recovery: After microwave treatment, cool the mixture to room temperature. Recover the product by centrifugation at 12,000 rpm for 15 minutes.
Washing and Purification: Wash the precipitate sequentially with deionized water and ethanol three times each to remove unreacted precursors and byproducts.
Drying and Annealing: Dry the product at 80°C for 12 hours in a vacuum oven. For enhanced crystallinity of the TiO₂ shell, anneal at 450°C for 2 hours in air (heating rate: 3°C/min) [43].
Advanced Protocol: Microwave-Assisted Vacuum Synthesis of TiOâ‚‚
For specialized applications requiring high crystallinity and controlled particle size, microwave-assisted vacuum synthesis offers distinct advantages:
Materials:
- Titanium tetraisopropoxide (Ti(O-iPr)â‚„, technical grade)
- Hydrochloric acid (HCl, 32% in water)
- Triton X-100 (laboratory grade)
- Isopropanol (technical grade)
- Bi-distilled water
Procedure:
- Acidic Mixture Preparation: Combine 77 mL of an acidic mixture containing HCl (3.100%), bi-distilled water (96.887%), and Triton X-100 (0.013%).
Precursor Addition: Add 25 mL Ti(O-iPr)â‚„ dropwise over 5 minutes to the acidic mixture under mechanical stirring (600 rpm).
Microwave-Vacuum Processing: Transfer the mixture to a modified microwave system equipped with vacuum distillation capability. Apply vacuum (approximately 10â»Â³ torr) and irradiate with microwave at maximum power of 500 W. Maintain temperature at 60-70°C for 25-40 minutes [44].
Product Collection: Depending on process duration, the product may be obtained as a thick white gel or dry powder. For powdered products, resuspend in bi-distilled water to achieve 6 wt% concentration of TiOâ‚‚.
Characterization: The resulting TiOâ‚‚ nanoparticles typically exhibit crystalline anatase phase with photocatalytic activity comparable to commercial standards [44].
Critical Parameters and Optimization
Microwave-assisted sol-gel synthesis requires careful control of several parameters to achieve reproducible results with desired properties:
Microwave Power and Irradiation Time: Optimal power levels (300-500 W) and irradiation times (20-40 minutes) depend on the specific material system. Excessive power or prolonged irradiation can lead to particle agglomeration and structural defects.
Temperature Control: Precise temperature monitoring is essential, as microwave heating can rapidly elevate temperatures beyond desired setpoints. Use vessels with integrated temperature sensors for real-time monitoring.
Pressure Conditions: Conventional microwave synthesis operates at atmospheric pressure, while advanced implementations may utilize vacuum or controlled pressure conditions to manipulate reaction kinetics and solvent evaporation rates [44].
Precursor Concentration and Solvent Selection: Higher precursor concentrations generally yield larger particles, while solvent polarity directly affects microwave absorption efficiency and heating rates.
Table 2: Optimization Parameters for Microwave-Assisted Sol-Gel Synthesis
| Parameter | Optimal Range | Influence on Product | Remarks |
|---|---|---|---|
| Microwave Power | 300-500 W | Affects crystallization kinetics and particle size | Higher power → faster crystallization |
| Irradiation Time | 20-40 minutes | Determines crystallinity and phase purity | Material-dependent optimization required |
| Temperature | 60-80°C | Controls reaction rate and nucleation density | Critical for metastable phase formation |
| Pressure | Atmospheric/Vacuum | Influences solvent evaporation and byproduct removal | Vacuum shifts equilibrium toward products [44] |
| Precursor Concentration | 0.1-0.5 M | Impacts particle size and size distribution | Higher concentration → larger particles |
Characterization and Performance Evaluation
Comprehensive characterization validates the efficacy of microwave-assisted approaches and guides further optimization:
Phase Identification and Crystallinity: XRD analysis confirms phase formation and crystallite size. Microwave-synthesized TiOâ‚‚ typically exhibits anatase phase with crystallite sizes of 10-30 nm. The rapid heating often results in unique phase distributions not easily achievable through conventional methods [44].
Morphological Analysis: SEM and TEM imaging reveal core-shell structures in nanocomposites, with uniform TiO₂ coating on Fe₂O³ cores typically measuring 2-5 nm in thickness. Electron diffraction confirms crystallinity of both core and shell components [43].
Elemental Composition and Surface Chemistry: X-ray photoelectron spectroscopy (XPS) verifies chemical states and confirms successful doping or composite formation. For Ag/TiO₂ systems, XPS shows Ag 3d peaks at 368.2 eV (3d₅/₂) and 374.2 eV (3d₃/₂), confirming metallic silver incorporation [47].
Optical Properties: UV-Vis spectroscopy demonstrates enhanced visible-light absorption in doped or composite systems. Fe₂O₃@TiO₂ core-shell structures show significant red-shift in absorption edge compared to pure TiO₂, extending photocatalytic activity into the visible spectrum [43].
Photocatalytic Performance: Evaluation through contaminant degradation (e.g., Cr(VI) reduction or organic dye decomposition) under appropriate light irradiation. Ag/TiOâ‚‚ synthesized via microwave assistance demonstrated 100% reduction of 24 ppm Cr(VI) within 60-120 minutes, outperforming pure TiOâ‚‚ references [47].
Comparative Analysis and Application Prospects
Technique Selection Guidelines
The choice between hydrothermal-assisted and microwave-assisted sol-gel methods depends on specific application requirements and material characteristics:
Hydrothermal-assisted sol-gel is preferable when:
- High crystallinity and well-defined crystal morphologies are prioritized
- Synthesis of complex multi-metal oxides is required
- Control over particle size distribution is critical
- Production of materials with high phase purity is essential
Microwave-assisted sol-gel is advantageous for:
- Rapid synthesis and high-throughput screening of materials
- Formation of metastable phases with unique properties
- Synthesis of composites and doped materials with homogeneous distribution
- Energy-efficient processing with reduced environmental impact
Table 3: Performance Comparison of Advanced Sol-Gel Synthesis Techniques
| Characteristic | Hydrothermal-Assisted | Microwave-Assisted | Conventional Sol-Gel |
|---|---|---|---|
| Processing Time | 12-48 hours | 20-60 minutes | 24-72 hours |
| Crystallinity | High | Moderate to High | Variable (requires calcination) |
| Particle Size Control | Excellent | Good | Moderate |
| Energy Consumption | Moderate | Low | High (with calcination) |
| Equipment Complexity | High (autoclave required) | Moderate | Low |
| Scalability | Challenging | Promising | Well-established |
| Typical Surface Area | 10-50 m²/g | 20-100 m²/g | 50-500 m²/g (before calcination) |
Applications in Photocatalysis and Beyond
Metal oxides synthesized via these advanced sol-gel methods find applications across diverse photocatalytic domains:
Environmental Remediation: TiOâ‚‚-based photocatalysts effectively degrade organic pollutants (dyes, pesticides, pharmaceuticals) and reduce toxic heavy metals (Cr(VI) to Cr(III)) in wastewater treatment systems [47].
Energy Conversion: Fe₂O₃@TiO₂ core-shell structures demonstrate enhanced visible-light activity for hydrogen production via water splitting, leveraging the complementary optical properties of both materials [43].
Air Purification: BaTiO₃ and related perovskites exhibit excellent performance in photocatalytic oxidation of volatile organic compounds (VOCs) for indoor air quality management [45] [19].
Antimicrobial Applications: The reactive oxygen species generated by these photocatalysts under light irradiation enable effective bacterial inactivation on surfaces and in water purification systems [48].
Beyond photocatalysis, these materials find applications in diverse fields including dielectric ceramics (BaTiO₃ for capacitors), sensors, biomedical imaging (Fe₂O₃ for magnetic resonance imaging), and energy storage (TiO₂ for lithium-ion batteries).
The Scientist's Toolkit
Table 4: Essential Research Reagent Solutions for Advanced Sol-Gel Synthesis
| Reagent/Chemical | Function | Application Examples | Handling Considerations |
|---|---|---|---|
| Titanium tetra-n-butoxide | Titanium precursor | TiOâ‚‚ nanoparticle synthesis | Moisture-sensitive; handle under inert atmosphere |
| Barium acetate | Barium source | BaTiO₃ perovskite synthesis | Requires acidic dissolution |
| Polyethylene glycol (PEG) | Structure-directing agent | Mesoporous structure control | Molecular weight affects pore size |
| Cetyltrimethylammonium chloride (CTAC) | Surfactant template | Mesoporous silica nanoparticles | Removal required post-synthesis |
| Ammonia solution (NHâ‚„OH) | Catalyst | Condensation reaction promotion | Concentration affects hydrolysis rates |
| Hydrochloric acid (HCl) | Catalyst | Acid-catalyzed hydrolysis | Controls reaction kinetics |
| Silver nitrate (AgNO₃) | Dopant precursor | Ag/TiO₂ plasmonic photocatalysts | Light-sensitive; requires dark storage |
| Ethylene glycol (EG) | Solvent/Reagent | High-boiling point solvent | Enables elevated temperature reactions |
| 1,2,4-Triazine, 5-phenyl- | 1,2,4-Triazine, 5-phenyl-, CAS:18162-28-2, MF:C9H7N3, MW:157.17 g/mol | Chemical Reagent | Bench Chemicals |
| Diethenyl ethanedioate | Diethenyl Ethanedioate|C6H6O4|Research Chemical | Research-grade Diethenyl Ethanedioate (C6H6O4). This product is For Research Use Only (RUO) and is not intended for personal use. | Bench Chemicals |
Workflow and Pathway Diagrams
Application Notes: Performance and Characteristics of Composite Photocatalysts
The strategic design of composite metal oxide photocatalysts via the sol-gel method significantly enhances their performance for environmental remediation and energy applications. By combining semiconductors like TiOâ‚‚ or ZnO with SiOâ‚‚ or other metal oxides, it is possible to tailor their physicochemical properties, leading to improved charge separation, enhanced stability, and greater surface area.
TiOâ‚‚-SiOâ‚‚ Composite Photocatalysts
Integrating SiO₂ with TiO₂ creates a composite material that benefits from the synergistic effects between its components. The SiO₂ matrix often inhibits the crystal growth of TiO₂, leading to a higher surface area and improved adsorption capacity for pollutants. Furthermore, the formation of Ti–O–Si bonds can modify the band structure, potentially reducing charge carrier recombination [30] [49].
Table 1: Performance and Characteristics of TiOâ‚‚-SiOâ‚‚ Photocatalysts
| Property / Performance Metric | TiOâ‚‚-SiOâ‚‚ Composite (Sol-Gel) | Pure TiOâ‚‚ (Sol-Gel) | Characterization Method | References |
|---|---|---|---|---|
| Photocatalytic Efficiency (Methylene Blue) | ~70% degradation in 5 h | Not Specified | UV-Vis Spectroscopy | [33] |
| Photocatalytic Efficiency (Rhodamine B) | ~92% degradation in 6 h | Not Specified | UV-Vis Spectroscopy | [33] |
| Point of Zero Charge (pHpzc) | 6.4 | Not Specified | Dye adsorption at different pH | [33] |
| Primary Crystalline Phase | Anatase/Rutile | Anatase | X-ray Diffraction (XRD) | [50] |
| Adhesion to Substrates | Enhanced | Reduced | Scanning Electron Microscopy (SEM) | [50] |
| BET Surface Area | 303 m²/g | ~60 m²/g (Commercial) | N₂ Adsorption-Desorption | [49] |
| Photoconductivity | ~300x increase (at 800 V/cm) | Baseline | Field-dependent measurement | [49] |
The point of zero charge (pHpzc) of 6.4 for the TiOâ‚‚-SiOâ‚‚ composite suggests it is effective for adsorbing cationic dyes like Methylene Blue (MB) and Rhodamine B (RhB) at near-neutral pH, facilitating their subsequent degradation [33]. While the addition of SiOâ‚‚ may marginally reduce the pure photocatalytic efficiency compared to pure TiOâ‚‚, it significantly enhances the coating's adhesion to substrates like ceramic tiles, which is critical for practical, immobilized photocatalytic applications [50].
ZnO-Based Composite Photocatalysts
ZnO is a widely studied alternative to TiOâ‚‚ due to its high exciton binding energy and efficient UV light absorption. Its photocatalytic performance is highly dependent on its morphology and crystallinity, which can be precisely controlled through sol-gel synthesis parameters, particularly the choice of solvent [17] [51].
Table 2: Performance and Characteristics of ZnO-Based Photocatalysts
| Property / Performance Metric | ZnO with Ethanol Solvent | ZnO with 1-Propanol Solvent | ZnO with 1,4-Butanediol Solvent | References |
|---|---|---|---|---|
| MB Degradation Efficiency | 98% (Rapid degradation) | Lower than Ethanol-based | Lower than Ethanol-based | [17] |
| Antibacterial Activity (S. aureus) | 30 mm (Nanorods, Z1) / 31 mm (Microspheres, Z2) | Not Specified | Not Specified | [51] |
| Antibacterial Activity (E. coli) | 30 mm (Nanorods, Z1) / 35 mm (Microspheres, Z2) | Not Specified | Not Specified | [51] |
| Crystallite Size | Not Specified | Not Specified | Not Specified | |
| Crystal Structure | Wurtzite (all) | Wurtzite | Wurtzite | [17] [51] |
The morphology of ZnO nanoparticles is a critical factor determining their biological activity. For instance, 3D microsphere-shaped ZnO particles demonstrated a larger zone of inhibition against E. coli (35 mm) compared to ellipsoidal nanorods (30 mm), which is attributed to their higher surface area and interaction with bacterial membranes [51]. This morphology-dependent activity highlights the importance of synthesis control for target applications.
Multi-Metal Oxide and TMO/GO Composite Systems
Advanced composite systems, such as transition metal oxide/graphene oxide (TMO/GO) nanocomposites, represent a further evolution in photocatalyst design. These materials combine the redox properties of TMOs with the exceptional electrical conductivity and high surface area of GO, leading to superior charge separation and multifunctional performance [52].
Table 3: Emerging Multi-Metal Oxide Photocatalysts for Dye Degradation and Energy Storage
| Composite System | Key Feature / Mechanism | Reported Performance | Potential Applications | References |
|---|---|---|---|---|
| Fe-doped Co₃Oâ‚„ | Bandgap reduction to ~2.0 eV; Co²âº/Co³⺠and Fe³⺠redox couples | High pseudocapacitance (588.5 F gâ»Â¹) | Photocatalysis, Supercapacitors | [52] |
| Co₃O₄-coated TiO₂ | Core-shell structure forming p-n junctions | ~100% MB degradation in 1.5 h under UV | Enhanced Photocatalysis | [52] |
| CuO/SnO₂ | p-n heterojunction; bandgap ~2.1–2.6 eV | Enhanced redox kinetics | Photocatalysis, Energy Storage | [52] |
| ZnO-SiOâ‚‚ (10:90) | Formation of Zn-O-Si bonds; amorphous SiOâ‚‚ matrix with ZnO crystallites | High surface area; potential for Hâ‚‚ storage | Photocatalysis, Optoelectronics | [30] |
These multi-metal systems leverage tunable bandgaps and engineered heterojunctions to enhance visible-light absorption and facilitate the separation of photogenerated electron-hole pairs, thereby boosting photocatalytic efficiency [52]. The integration with graphene oxide further addresses intrinsic limitations of TMOs, such as poor electrical conductivity and particle agglomeration [52].
Experimental Protocols
This section provides detailed, reproducible methodologies for the synthesis and evaluation of key composite photocatalysts, as referenced in the application notes.
Protocol 1: Synthesis of TiOâ‚‚-SiOâ‚‚ Mixed Oxide via Sol-Gel
This protocol is adapted from studies demonstrating high photocatalytic degradation efficiency for dyes like Rhodamine B and Methylene Blue [33].
Research Reagent Solutions
| Reagent / Material | Function in Synthesis |
|---|---|
| Titanium isopropoxide (TTIP) | TiOâ‚‚ precursor |
| Tetraethyl orthosilicate (TEOS) | SiOâ‚‚ precursor |
| Isopropyl alcohol | Solvent |
| 0.1 M HCl & 0.1 M NaOH | pH adjustment |
| Deionized Water | Hydrolysis agent |
Step-by-Step Procedure:
- Solution A (TiOâ‚‚ Sol): Slowly add 0.0101 mol of TTIP dropwise into isopropyl alcohol under constant stirring. Stir for 30 minutes.
- Solution B (SiOâ‚‚ Sol): Add 0.0224 mol of TEOS dropwise into a separate container with isopropyl alcohol. Stir for 30 minutes.
- Mixing: Combine Solution A and Solution B in a 1:1 volume ratio to form the mixed oxide sol. Continue stirring.
- Gelation: Induce gelation by adjusting the pH of the mixture using 0.1 M NH₃ solution (as per similar protocol [49]). Alternatively, pH control can be maintained with 0.1 M HCl or NaOH depending on the desired kinetics.
- Aging and Drying: Age the resulting gel for 24 hours. Filter the gel and wash several times with deionized water to remove by-products. Dry the product in an oven at 80°C for 24 hours.
- Calcination: Calcine the dried powder in a muffle furnace at 500°C for 3 hours to crystallize the TiO₂ phase, primarily into anatase/rutile [33] [50].
Protocol 2: Sol-Gel Synthesis of ZnO Nanoparticles with Solvent Variation
This protocol, based on a study achieving 98% MB degradation, highlights the critical role of the solvent [17].
Research Reagent Solutions
| Reagent / Material | Function in Synthesis |
|---|---|
| Zinc acetate dihydrate | ZnO precursor |
| Oxalic acid dihydrate | Gelating agent |
| Ethanol, 1-Propanol, 1,4-Butanediol | Solvents for variation study |
Step-by-Step Procedure:
- Preparation of Pot 1: Dissolve a measured amount of zinc acetate dihydrate in 50 mL of a selected solvent (e.g., ethanol). Stir at 50°C for 30 minutes using a magnetic stirrer.
- Preparation of Pot 2: Dissolve an equimolar amount of oxalic acid in 25 mL of the same solvent at room temperature.
- Reaction and Gelation: Gradually add the contents of Pot 2 to Pot 1. Continue stirring the mixture at 70°C until a viscous white gel is formed. The reaction produces zinc oxalate.
- Drying: Transfer the gel to an oven and dry at 80°C overnight.
- Calcination: Calcine the dried zinc oxalate powder at 600°C for 4 hours in air to thermally decompose it into ZnO nanoparticles.
- Repeat: Repeat the entire process using 1-propanol and 1,4-butanediol as solvents to produce samples for comparative analysis.
Protocol 3: Synthesis of ZnO-SiOâ‚‚ Nanocomposite (10:90)
This protocol focuses on creating a composite with intimate contact between ZnO and SiOâ‚‚, evidenced by Zn-O-Si bond formation [30].
Research Reagent Solutions
| Reagent / Material | Function in Synthesis |
|---|---|
| Tetraethyl orthosilicate (TEOS) | SiOâ‚‚ precursor |
| Zinc acetate dihydrate | ZnO precursor |
| Ethanol | Solvent |
| Acetic acid | Catalyst for SiOâ‚‚ synthesis |
| 0.03 M HCl | Prevents Zn(OH)â‚‚ precipitation |
Step-by-Step Procedure:
- SiO₂ Sol Preparation: Mix TEOS, ethanol, and deionized water in a molar ratio of 1:10:4. Add 0.05 mol acetic acid (per mol TEOS) as a catalyst. Stir vigorously for 3 hours at 25°C.
- Gelation and Aging: Age the sol for 18 hours at 75°C to form a gel. Dry the SiO₂ gel at 120°C.
- ZnO-SiOâ‚‚ Composite: a. Prepare a solution of zinc acetate dihydrate in ethanol. b. Incorporate this solution into the SiOâ‚‚ sol prior to its gelation, targeting a 10% wt. ZnO final composition. c. Add 0.03 M HCl to the mixture to prevent the precipitation of zinc hydroxide. d. Stir the mixture thoroughly to ensure homogeneity.
- Drying and Annealing: Dry the composite gel and then anneal it at temperatures ranging from 700°C to 900°C to crystallize the ZnO phase while the SiO₂ matrix remains largely amorphous.
Protocol 4: Standardized Photocatalytic Activity Test
This is a generalized procedure for evaluating dye degradation performance, consistent across multiple studies [33] [17].
Step-by-Step Procedure:
- Reaction Setup: Prepare an aqueous solution of the target organic dye (e.g., Methylene Blue) with a concentration of 5 mg/L. Use 100 mL of this solution for each test.
- Catalyst Loading: Add 0.1 g of the synthesized photocatalyst powder to the dye solution.
- Dark Adsorption: Stir the mixture in the dark for 30-60 minutes to establish adsorption-desorption equilibrium.
- Irradiation: Place the reaction mixture under a light source (e.g., two Philips TL 8 W BLB UV lamps positioned 15 cm above the solution) while maintaining constant magnetic stirring.
- Sampling and Analysis: At regular time intervals, withdraw small aliquots of the solution. Centrifuge them to remove the catalyst particles.
- Measurement: Analyze the clear supernatant using UV-Vis spectroscopy by measuring the absorbance at the characteristic maximum wavelength of the dye (e.g., 664 nm for MB). The degradation efficiency is calculated as (C₀ - Cₜ)/C₀ × 100%, where C₀ and Cₜ are the initial concentration and concentration at time t, respectively.
Visualization of Synthesis and Mechanism
The following diagrams illustrate the core synthesis workflow and the functional mechanism of the composite photocatalysts.
Sol-Gel Synthesis Workflow for Metal Oxide Composites
Photocatalytic Mechanism in a TiOâ‚‚-SiOâ‚‚ Composite
In the broader context of synthesizing metal oxide photocatalysts via the sol-gel method, engineering efficient charge separation stands as a central challenge. The sol-gel technique offers unparalleled control over composition and microstructure, making it ideal for fabricating advanced heterostructures [7] [6]. This document details the application of sol-gel synthesized materials in two primary charge separation systems: p-n junctions and Z-scheme heterojunctions. These architectures are critical for enhancing photocatalytic performance in environmental remediation and energy applications, directly supporting the core thesis that sol-gel processing is a versatile platform for designing next-generation photocatalysts.
The fundamental limitation in photocatalysis is the rapid recombination of photogenerated electron-hole pairs, which reduces quantum efficiency. Heterojunction engineering provides a solution by creating internal electric fields or tailored charge transfer pathways that spatially separate these charge carriers [53]. This note provides detailed protocols for creating and characterizing these systems, summarizes key performance data, and outlines essential reagents and experimental workflows.
Heterojunction Mechanisms and Experimental Evidence
p-n Junction Systems
Principle of Operation: A p-n junction is formed by the intimate contact of a p-type semiconductor and an n-type semiconductor. Due to the difference in Fermi levels, electrons diffuse from the n-type to the p-type material, and holes diffuse in the opposite direction upon contact. This redistribution creates a built-in internal electric field in the space-charge region, which drives the separation of photogenerated charge carriers under illumination [53].
Sol-Gel Synthesis Evidence: The sol-gel method is highly effective for creating homogeneous p-n heterojunctions. Research on Zn0.75Ni0.25Fe2O4 demonstrates this principle, where the substitution of cations creates a system that functions as a p-n junction, as confirmed by Diffuse Reflectance Spectroscopy (DRS) [54]. The internal electric field in this structure significantly enhances the separation of electrons and holes (eâ»/h⺠pairs), leading to superior photocatalytic performance.
Table 1: Performance Data for a Sol-Gel Synthesized p-n Junction Photocatalyst (Zn₀.₇₅Ni₀.₂₅Fe₂O₄)
| Property | Value | Measurement Method |
|---|---|---|
| Bandgap Energy | 1.59 eV | DRS [54] |
| Crystallite Size | 20.4 nm | Williamson-Hall model [54] |
| Photocatalytic Efficiency | 86.4% (Metronidazole degradation) | Degradation test under optimized conditions [54] |
| Optimal Catalyst Dose | 0.8 g/L | Performance optimization [54] |
| Optimal pH | 5 | Performance optimization [54] |
Z-Scheme Systems
Principle of Operation: Z-scheme heterojunctions mimic natural photosynthesis. In a typical direct Z-scheme, two semiconductors are coupled without a redox mediator. The photogenerated electrons from the higher conduction band combine with holes from the lower valence band at the interface. This selective recombination preserves the most energetic electrons in one semiconductor and the most powerful holes in the other, achieving both high charge separation efficiency and strong redox ability [55] [56].
Experimental Validation: Techniques to confirm the Z-scheme mechanism include:
- Radical Trapping Experiments: Using specific scavengers to identify which reactive species (e.g., •OH, •Oâ‚‚â») are responsible for degradation [57].
- X-ray Photoelectron Spectroscopy (XPS): Detecting shifts in binding energy that indicate electron transfer direction at the interface [57].
- DFT Calculations: Theoretically modeling band structures and charge density differences to predict and verify charge transfer pathways [57].
Table 2: Performance of a Z-Scheme Heterojunction (Z₀.₆C₀.₄S/ZnO/CN-35%)
| Pollutant | Degradation Efficiency | Time | Key Radicals |
|---|---|---|---|
| Methylene Blue (MB) | 98.52% | Under visible light | •OH, •O₂⻠[57] |
| Rhodamine B (RhB) | 99.45% | Under visible light | •OH, •O₂⻠[57] |
| Tetracycline (TC) | 98.20% | Under visible light | •OH, •O₂⻠[57] |
Experimental Protocols
Protocol 1: Sol-Gel Synthesis of a p-n Junction Photocatalyst
This protocol outlines the synthesis of a Zn-Ni ferrite-based p-n junction photocatalyst for antibiotic degradation [54].
Research Reagent Solutions:
- Precursor Salts: Zinc nitrate hexahydrate (Zn(NO₃)₂·6H₂O) and Nickel nitrate hexahydrate (Ni(NO₃)₂·6H₂O) as metal cation sources.
- Iron Precursor: Iron(III) nitrate nonahydrate (Fe(NO₃)₃·9H₂O).
- Gelling Agent: Citric acid (C₆H₈O₇) as a complexing agent and fuel for combustion.
- Solvent: Deionized water.
Procedure:
- Solution Preparation: Dissolve stoichiometric amounts of Zn(NO₃)₂·6H₂O, Ni(NO₃)₂·6H₂O, and Fe(NO₃)₃·9H₂O in deionized water using a 1:3 molar ratio of Zn:Ni to achieve the final composition of Zn₀.₇₅Ni₀.₂₅Fe₂O₄ [54].
- Complexation: Add an equimolar amount of citric acid to the total metal cations in the solution. Stir vigorously at 70-80°C until a homogeneous sol forms.
- Gelation: Continue heating the sol at 120°C with constant stirring until it transforms into a viscous gel.
- Combustion and Calcination: Ignite the dried gel in a preheated muffle furnace at 300°C for 2 hours. Subsequently, calcine the resulting powder at 600°C for 4 hours to crystallize the Zn₀.₇₅Ni₀.₂₅Fe₂O₄ spinel phase.
- Characterization: Characterize the final powder using XRD for phase identification and crystallite size, SEM for morphology, and DRS for bandgap determination [54].
Protocol 2: Fabrication of a Ternary Z-Scheme Heterostructure
This protocol describes the construction of a Zn₀.₆Cd₀.₄S/ZnO/g-C₃N₄ dual Z-scheme heterostructure via a combined calcination-hydrothermal method [57].
Research Reagent Solutions:
- g-C₃N4 Precursor: Melamine (C₆H₆N₆) for synthesizing graphitic carbon nitride.
- Metal Sources: Zinc acetate dihydrate (Zn(CH₃COO)₂·2H₂O) and Cadmium acetate dihydrate (Cd(CH₃COO)₂·2H₂O).
- Sulfur Source: Thiourea (CHâ‚„Nâ‚‚S).
- Structure Director: Polyvinylpyrrolidone (PVP).
- pH Modifier: Sodium hydroxide (NaOH) solution.
Procedure:
- Synthesis of g-C₃N₄: Place 10 g of melamine in an alumina crucible and calcine in a muffle furnace at 550°C for 4 hours with a ramp rate of 18°C/min. After cooling, a faint yellow g-C₃N₄ powder is obtained [57].
- Precursor Mixing:
- Dissolve Zn(CH₃COO)₂·2H₂O (0.2177 g) and Cd(CH₃COO)₂·2H₂O (0.1763 g) in 10 mL deionized water (Solution A).
- Dissolve CHâ‚„Nâ‚‚S (0.1586 g) in a mixture of 10 mL deionized water and 10 mL anhydrous ethanol (Solution B).
- Mix Solutions A and B under magnetic stirring.
- Add a NaOH solution to adjust the pH to 7 and stir for 40 minutes.
- Hydrothermal Synthesis: Add a specific mass of g-C₃N₄ (e.g., 0.05 g for a 25% composite) and 0.2 g of PVP to the mixed solution. Transfer the solution to a 100 mL Teflon-lined autoclave and maintain it at 180°C for 24 hours.
- Post-processing: After cooling, wash the resulting product three times with deionized water and anhydrous ethanol. Dry the final Zn₀.₆Cd₀.₄S/ZnO/g-C₃N₄ catalyst in a vacuum oven at 80°C for 12 hours [57].
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for Sol-Gel Heterojunction Synthesis
| Reagent / Material | Function in Synthesis | Example Application |
|---|---|---|
| Tetraethyl Orthosilicate (TEOS) | Precursor for SiOâ‚‚ matrix; provides structural support and inhibits aggregation [6]. | ZnO-SiOâ‚‚ nanocomposites [6]. |
| Zinc Acetate Dihydrate | Precursor for ZnO nanoparticles; favored for producing smooth surfaces [6]. | ZnO-based heterojunctions [6] [57]. |
| Citric Acid | Chelating agent in sol-gel process; controls polymerization and acts as a fuel in combustion synthesis [54]. | NiFeâ‚‚Oâ‚„ and ZnFeâ‚‚Oâ‚„ synthesis [54]. |
| Urea (NHâ‚‚CONHâ‚‚) | Fuel for combustion synthesis; hydrolyzing agent that influences final morphology and crystal structure [58]. | CaO:MgAlâ‚‚Oâ‚„ nanocomposite [58]. |
| Melamine | Precursor for graphitic carbon nitride (g-C₃N₄), a non-metallic photocatalyst [57]. | Z-scheme heterostructures [57]. |
| Polyvinylpyrrolidone (PVP) | Capping agent or structure director; controls particle growth and prevents agglomeration [57]. | Zn₀.₆Cd₀.₄S/ZnO/g-C₃N₄ composite [57]. |
| Cobalt--dysprosium (1/3) | Cobalt--dysprosium (1/3), CAS:12200-33-8, MF:CoDy3, MW:546.43 g/mol | Chemical Reagent |
| Diethyl hex-2-enedioate | Diethyl Hex-2-enedioate|CAS 21959-75-1|RUO |
Workflow and Charge Transfer Pathways
The following diagrams illustrate the experimental workflow for heterojunction fabrication and the fundamental charge separation mechanisms in p-n and Z-scheme systems.
Diagram 1: Sol-gel synthesis workflow for heterojunction fabrication.
Diagram 2: Charge transfer mechanisms in p-n and Z-scheme heterojunctions.
The sol-gel method has emerged as a powerful synthetic route for designing metal oxide photocatalysts with tailored properties for environmental remediation, energy conversion, and biomedical applications. A principal challenge in photocatalyst development lies in the inherent wide bandgap of many metal oxides, such as TiO₂ (∼3.2 eV) and ZnO (∼3.37 eV), which restricts their photoabsorption primarily to the ultraviolet region, representing only a small fraction of the solar spectrum [59] [60]. Bandgap engineering through strategic doping with transition metals and non-metals provides a versatile approach to modulate the electronic structure, enhance visible-light absorption, and ultimately improve photocatalytic efficiency [7]. The sol-gel process is particularly suited for this purpose, as it allows for molecular-level mixing of precursors, facilitating homogeneous dopant incorporation and controlled crystallization at relatively low temperatures [3] [6]. This application note details the principles, protocols, and analytical methods for the functionalization of metal oxide photocatalysts via doping, contextualized within a broader research thesis on sol-gel synthesis.
The fundamental principle involves introducing foreign atoms into the host oxide lattice to create new electronic states within the bandgap. Transition metals, with their distinct d-electron configurations, typically introduce states within the gap, effectively narrowing it and enhancing visible light absorption [61] [59]. For instance, early transition metals like Ti and V are known to reduce the bandgap and enhance charge carrier mobility [61]. Non-metal dopants (e.g., N, C, S), on the other hand, often modify the valence band by mixing their p orbitals with the O 2p orbitals, thereby raising the valence band maximum and reducing the bandgap [62] [63]. The choice of dopant, its concentration, and its spatial distribution are critical parameters that dictate the optical, electronic, and catalytic properties of the resulting material [7] [60].
Theoretical Foundations and Doping Strategies
Bandgap Tuning Mechanisms
Doping modulates the bandgap of metal oxides through several interconnected mechanisms. The s-d exchange interaction between the conduction band electrons (s) and the localized d-electrons of transition metal dopants can lead to a significant reduction in the bandgap energy (Eg), as described by microscopic models and confirmed by experimental red-shifts in absorption spectra [59]. This interaction is also responsible for inducing room-temperature ferromagnetism in certain dilute magnetic semiconductors.
For non-metal dopants, the formation of charged defects is a crucial factor. Contrary to early assumptions that neutral defects dominate, advanced theoretical studies using hybrid DFT indicate that charged defects, such as N substitution (trapping one electron) or Se interstitial (trapping two holes), can be the most thermodynamically stable configurations under realistic temperature and oxygen partial pressures [63]. These charged states effectively create intra-gap levels that facilitate sub-bandgap photon absorption.
Furthermore, doping often induces lattice strain and distortions. The substitution of host cations or anions with dopants of different ionic radii leads to changes in lattice constants and bond lengths. This strain subsequently affects the exchange interaction constants within the lattice, contributing to bandgap modulation [64] [59]. For example, in α-NiS, doping with various transition metals increases lattice constants, which correlates with a reduction in the bandgap [64].
Strategic Selection of Dopants
The rational selection of dopants is guided by the desired electronic and functional outcomes.
- Early Transition Metals (e.g., Ti, V): These dopants are effective in significantly lowering the bandgap and enhancing electrical conductivity and charge mobility, which is beneficial for catalytic applications [61].
- Mid-Transition Metals (e.g., Fe, Co): These elements often provide a balance between bandgap reduction and the maintenance of structural integrity, making them ideal for stable catalytic systems [61] [64]. Fe and Co doping in α-NiS, for example, drastically enhance charge carrier mobility and separation [64].
- Late Transition Metals (e.g., Pd, Ag): These dopants are known to create highly conductive pathways, offer significant bandgap reduction, and improve oxidative potential via enhanced electron transfer [61].
- Non-Metals (e.g., N, C, S, Se): These are favored for avoiding the formation of recombination centers common with some metal dopants. They enhance visible-light response by modifying the valence band and can form stable charged defects [62] [63]. Co-doping with both metals and non-metals (e.g., Al/S in TiOâ‚‚) can create synergistic effects, leading to dramatic bandgap narrowing and improved charge separation [60].
Table 1: Quantitative Effects of Selected Dopants on Bandgap and Photocatalytic Performance
| Host Material | Dopant(s) | Bandgap (eV) | Photocatalytic Performance | Reference |
|---|---|---|---|---|
| TiOâ‚‚ | None (Pure) | 3.23 | 15% MB degradation in 150 min | [60] |
| TiOâ‚‚ | Al (2%), S (8%) | 1.98 | 96.4% MB degradation in 150 min | [60] |
| α-NiS | None (Pure) | — | Baseline | [64] |
| α-NiS | Fe (25%) | 1.10 (Theoretical) | High carrier mobility & separation | [64] |
| TiO₂-SiO₂ Composite | None (Composite) | — | ~92% RhB degradation in 6 h; ~70% MB degradation in 5 h | [33] |
| Graphitic Carbon Nitride | Early TMs (e.g., Ti, V) | Bandgap reduced | Enhanced conductivity for catalysis | [61] |
Experimental Protocols: Sol-Gel Synthesis and Doping
General Sol-Gel Workflow for Doped Metal Oxides
The following diagram illustrates the generalized experimental workflow for the sol-gel synthesis of doped metal oxide photocatalysts, integrating key steps from multiple protocols.
Detailed Protocol: Synthesis of Al/S Co-Doped TiOâ‚‚ Nanoparticles
This protocol is adapted from recent research achieving a significant bandgap reduction to 1.98 eV [60].
3.2.1 Research Reagent Solutions
Table 2: Essential Reagents for Al/S Co-Doped TiOâ‚‚ Synthesis
| Reagent | Function/Note | Exemplary Quantity |
|---|---|---|
| Titanium (III) chloride hexahydrate (TiCl₃·6H₂O) | Primary TiO₂ precursor | 2 g in 50 mL DI Water |
| Aluminum (III) chloride hexahydrate (AlCl₃·6Hâ‚‚O) | Source of Al³âº/Al²⺠dopant ions | Molar ratio: 2% Al/Ti |
| Thiourea (SC(NHâ‚‚)â‚‚) | Source of Sâ¶âº dopant ions | Molar ratio: 2-8% S/Ti |
| Sodium Hydroxide (NaOH) | Precipitation and pH control agent | 0.5 g in 20 mL DI Water |
| Ammonium Hydroxide (NHâ‚„OH) | For pH adjustment to ~9 | q.s. |
| Deionized Water | Solvent | 70+ mL |
3.2.2 Step-by-Step Procedure
- Precursor Solution Preparation: Dissolve 2 g of TiCl₃·6H₂O in 50 mL of deionized water using a magnetic stirrer for 30 minutes to ensure complete dissolution.
- Dopant Addition: To the titanium precursor solution, add calculated amounts of AlCl₃·6H₂O and thiourea to achieve the desired dopant concentrations (e.g., 2% Al with 2-8% S relative to Ti moles). Stir the mixture vigorously for an additional 30 minutes to ensure a homogeneous solution.
- Precipitation and pH Adjustment: In a separate beaker, dissolve 0.5 g of NaOH in 20 mL of deionized water. Add this NaOH solution dropwise to the stirred Ti/Al/S mixture using a pipette. Subsequently, adjust the pH of the final solution to approximately 9 using ammonium hydroxide. This step facilitates uniform co-precipitation.
- Hydrothermal Reaction: Transfer the resulting solution into a 100 mL Teflon-lined stainless-steel autoclave. Seal the autoclave and place it in an oven. Heat the reaction mixture at 150 °C for 24 hours.
- Washing and Drying: After the reaction, allow the autoclave to cool naturally. Recover the precipitate via centrifugation. Wash the precipitate repeatedly with deionized water and ethanol until the supernatant reaches a neutral pH (pH ≈ 7). Transfer the washed product to a beaker and dry it in an oven at 60 °C for 24 hours.
- Calcination: To achieve crystallinity and complete dopant incorporation, calcine the dried powder in a muffle furnace at 500 °C for 3 hours in air, using a controlled heating ramp of 5 °C per minute.
Detailed Protocol: Synthesis of a TiOâ‚‚-SiOâ‚‚ Composite Photocatalyst
This protocol outlines the synthesis of a composite material, which is another effective strategy for modifying photocatalytic properties [33] [6].
3.3.1 Reagents
- TiOâ‚‚ Sol Precursor: Titanium isopropoxide (TTIP, 0.0101 mol).
- SiOâ‚‚ Sol Precursor: Tetraethyl orthosilicate (TEOS, 0.0224 mol).
- Solvent: Ethanol.
- Catalyst: Acetic acid or HCl/NaOH for pH control.
3.3.2 Step-by-Step Procedure
- Individual Sol Preparation:
- TiOâ‚‚ Sol: Hydrolyze TTIP (0.0101 mol) in ethanol under acidic catalysis (e.g., a few drops of HCl).
- SiOâ‚‚ Sol: Hydrolyze TEOS (0.0224 mol) in ethanol, using an ethanol-to-TEOS ratio of 10:1, with acetic acid as a catalyst (0.05 mol per mol TEOS) [6]. Stir for 3 hours.
- Mixing: Combine the TiOâ‚‚ and SiOâ‚‚ sols in a 1:1 volume ratio and stir vigorously to form a homogeneous mixed oxide sol.
- Gelation and Ageing: Allow the mixed sol to gel at 75 °C. Age the resulting gel for 18 hours to strengthen the metal-oxygen network.
- Drying and Annealing: Dry the aged gel at 120 °C to remove solvents. Finally, anneal the material at a temperature range of 500-700 °C to develop the final composite structure and crystallize the TiO₂ phase.
Material Characterization and Performance Evaluation
Structural and Chemical Analysis
- X-Ray Diffraction (XRD): Used to determine phase composition (anatase, rutile, brookite for TiOâ‚‚), crystallite size, and any lattice strain induced by dopants. Peak broadening and shifts confirm successful dopant incorporation [6] [60].
- Fourier-Transform Infrared (FT-IR) Spectroscopy: Identifies functional groups and chemical bonds. The formation of characteristic bands (e.g., Ti–O, Si–O, and crucially, M–O–Si in composites) and shifts in their positions provide evidence of dopant integration and composite formation [33] [6].
- Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy (SEM-EDS): Reveals surface morphology and provides semi-quantitative elemental analysis, confirming the presence and homogeneous distribution of dopant elements within the host material [33] [6].
- Electron Spin Resonance (ESR): Detects paramagnetic centers, such as Ti³⺠and oxygen vacancies, which are critical defect states often introduced by doping and responsible for enhanced photocatalytic activity and magnetic properties [60].
Optical and Electronic Properties
- UV-Visible Spectroscopy: The most direct method for evaluating bandgap tuning. Measure the diffuse reflectance or absorption spectrum and use the Tauc plot method to determine the optical bandgap. A redshift in the absorption edge indicates a successful reduction of the bandgap [33] [60].
- Photoluminescence (PL) Spectroscopy: Probes the fate of photogenerated charge carriers. A reduction in PL intensity for doped samples suggests a lower rate of electron-hole recombination, which is highly desirable for photocatalysis [60].
Functional Performance Assessment
- Photocatalytic Degradation:
- Prepare an aqueous solution of a model organic pollutant (e.g., Methylene Blue (MB) or Rhodamine B (RhB)) at a specific concentration (e.g., 10-20 mg/L).
- Add a precise amount of the photocatalyst powder (e.g., 1 g/L) to the solution and stir in the dark for 30-60 minutes to establish adsorption-desorption equilibrium.
- Illuminate the mixture under a visible light source (e.g., a Xe lamp with a UV cutoff filter). Maintain constant stirring and temperature.
- At regular time intervals, withdraw aliquots, centrifuge to remove catalyst particles, and analyze the supernatant using UV-Vis spectroscopy to monitor the degradation of the pollutant's characteristic absorption peak.
- Calculate the degradation efficiency and fit the kinetics to a pseudo-first-order model (Langmuir-Hinshelwood) to determine the rate constant, k [33] [60].
- Point of Zero Charge (pHâ‚šzc) Determination: The pHâ‚šzc is a crucial surface property. It can be determined by measuring the adsorption of charged dye molecules (e.g., Rhodamine B, Methylene Blue) at different initial pH levels. The pH at which adsorption is minimal is identified as the pHâ‚šzc (e.g., found to be 6.4 for a TiOâ‚‚-SiOâ‚‚ composite) [33].
The proliferation of organic contaminants, including industrial dyes, pharmaceutical products, and agricultural herbicides, in water systems poses a significant threat to ecosystems and human health [65] [66]. These pollutants are characterized by their persistence, low biodegradability, and potential to cause detrimental effects even at low concentrations. Advanced Oxidation Processes (AOPs), particularly heterogeneous photocatalysis, have emerged as proficient, green techniques for mineralizing these persistent organic pollutants into harmless substances such as water and carbon dioxide [67] [66].
The sol-gel method for synthesizing metal oxide photocatalysts is a cornerstone of this application due to its ability to produce materials with high specific surface area, controlled nanostructure, and tunable surface properties under mild conditions [65] [9]. This soft chemistry approach allows for the precise incorporation of dopants and the formation of composite structures, which are critical for enhancing photocatalytic efficiency. This document provides detailed application notes and experimental protocols for using sol-gel derived metal oxide photocatalysts in the degradation of dyes, pharmaceuticals, and herbicides, serving as a practical guide for researchers and scientists in the field of environmental remediation.
Photocatalytic Degradation: Mechanism and Workflow
The photocatalytic degradation of organic pollutants is initiated when a photon with energy equal to or greater than the semiconductor's band gap is absorbed, promoting an electron ((e^-)) from the valence band (VB) to the conduction band (CB). This process creates a positive hole ((h^+)) in the valence band, resulting in an electron-hole pair [65] [9] [67]. These photo-generated charge carriers migrate to the catalyst surface where they drive redox reactions. The holes can oxidize water molecules or hydroxide ions to generate highly reactive hydroxyl radicals ((•OH)), while the electrons can reduce adsorbed oxygen to form superoxide radical anions ((•O2^-)) [65] [9]. These radical species are non-selective and powerful oxidizing agents that subsequently attack and mineralize organic pollutants into benign products like (CO2) and (H_2O) [67].
Diagram 1: Photocatalytic mechanism for pollutant degradation.
A major challenge in photocatalysis is the rapid recombination of photogenerated electron-hole pairs, which can occur in nanoseconds, thus limiting the quantum efficiency of the process [65] [67]. Sol-gel synthesis offers a powerful strategy to mitigate this through metal ion doping (e.g., Ag, Fe, Mn) and composite formation (e.g., TiO₂-CeO₂, SiO₂–TiO₂/SnO₂). These modifications introduce electron traps, extend light absorption into the visible range, and provide more active sites for reactions [65] [68] [69].
Application Notes: Performance of Sol-Gel Photocatalysts
The following tables summarize the performance of various sol-gel synthesized metal oxide photocatalysts in degrading different classes of pollutants, as reported in recent literature.
Table 1: Degradation of Pharmaceutical Products and PPCPs
| Photocatalyst | Target Pollutant(s) | Experimental Conditions | Degradation Efficiency / Rate | Key Findings | Ref. |
|---|---|---|---|---|---|
| Ag NP-doped TiOâ‚‚ (Organic route) | 15 different pharmaceuticals | Thin film, UV light | Best overall performance | Required calcination; outperformed aqueous routes. | [65] |
| SiO₂–TiO₂/SnO₂ (SnCl₄ precursor) | Tetracycline (TC), Ciprofloxacin (CIP) | Visible light | TC degradation: 43.3x higher than pristine TiO₂ | SiO₂ incorporation improved performance 2.65-fold; enhanced charge separation. | [69] |
| Hybrid sol-gel/P25 TiOâ‚‚ | Oxytetracycline, Marbofloxacin, Ibuprofen, etc. | Glass beads, compact reactor | Complete mineralization achieved | Slower rates in wastewater vs. deionized water; acidic pH favored degradation. | [70] |
Table 2: Degradation of Dyes and Herbicides
| Photocatalyst | Target Pollutant(s) | Experimental Conditions | Degradation Efficiency / Rate | Key Findings | Ref. |
|---|---|---|---|---|---|
| ZnO Nanoparticle Thin Films | Methylene Blue (MB) | UV Light & UV Laser (305 nm) | MB monomer: 26% in 24h (UV), 72% in 3h (Laser) | Laser irradiation effectively degraded resistant MB dimers. | [71] |
| MnOâ‚‚ NP-doped TiOâ‚‚ | Methylene Blue (MB) | Thin film, UV light | High degradation efficiency | One of the top two candidates for MB degradation. | [65] |
| 0.05 wt% Fe₂O₃-doped TiO₂ | 2,4-Dichlorophenoxyacetic acid (2,4-D) | 1.0 g/L catalyst, pH 4, UVA lamp | 48% degradation in 240 min | Optimal doping; higher Fe content increased recombination. | [72] |
| TiO₂–CeO₂ | 2,4-Dichlorophenoxyacetic acid (2,4-D) | Annealing temp. 473-873 K | Efficiency dependent on annealing temperature | Higher temperature increased crystallite size but reduced surface area. | [68] |
Detailed Experimental Protocols
Protocol 1: Synthesis of Fe₂O₃-Doped TiO₂ via Aqueous Sol-Gel Route for Herbicide Degradation
This protocol is adapted from a study focusing on the optimization of 2,4-D herbicide degradation [72].
Research Reagent Solutions & Materials:
- Titanium(IV) butoxide (Precursor for TiOâ‚‚)
- Fe(NO₃)₃·9H₂O (Dopant precursor for Fe₂O₃)
- Absolute Ethanol (Solvent)
- Oxalic acid (0.1 mol/dm³) (Precipitating/Gelling agent)
- 0.1 M NaOH and HCl solutions (For pH adjustment)
- Deionized distilled water
Step-by-Step Methodology:
- Sol Preparation: Dissolve a stoichiometric amount of
Fe(NO₃)₃·9H₂Oin absolute ethanol. In a separate container, prepare a 0.1 M solution of titanium tert-butoxide in ethanol. Slowly mix the iron-containing solution with the titanium alkoxide solution under constant stirring. Continue stirring for 1 hour to ensure homogeneity. - Gel Formation and Aging: Slowly add a 0.1 M ethanolic solution of oxalic acid dropwise into the mixture to precipitate a thick yellowish-white gel.
- Purification: Purify the gel via centrifugation at 4000 rpm for multiple cycles (typically 3) using ethanol as the washing solvent.
- Drying and Calcination: Dry the purified gel in an oven at 70°C. Grind the resulting powder and calcine it in a tube furnace at 550°C for 4 hours to crystallize the anatase phase of TiO₂.
Photocatalytic Testing Setup:
- Reactor: Use a batch photoreactor equipped with a UVA lamp (e.g., 6W, maximum intensity at 365 nm).
- Reaction Mixture: Prepare a solution of the target pollutant (e.g., 10-50 ppm 2,4-D). Adjust the pH to the desired value (e.g., pH 4) using 0.1 M NaOH or HCl.
- Procedure: Add a predetermined mass of the photocatalyst (e.g., 0.2-1.0 g/L) to the pollutant solution. Aerate and stir the mixture continuously in the dark for 1 hour to establish adsorption-desorption equilibrium.
- Irradiation and Sampling: Turn on the UVA lamp to initiate the reaction. Withdraw aliquots (e.g., 10 mL) at regular intervals. Separate the catalyst from the solution by centrifugation or filtration before analysis.
- Analysis: Monitor the degradation by measuring the residual concentration of the pollutant using UV-Vis spectrophotometry (for 2,4-D, at λmax = 284 nm). The degradation efficiency ((\eta)) can be calculated as: (\eta (\%) = (C0 - Ct)/C0 \times 100), where (C0) and (C_t) are the initial concentration and concentration at time (t), respectively.
Protocol 2: Synthesis of ZnO Nanoparticle Thin Films via Sol-Gel for Dye Degradation
This protocol details the preparation of ZnO thin films for the enhanced degradation of Methylene Blue (MB) under laser irradiation [71].
Research Reagent Solutions & Materials:
- Zinc acetate dihydrate (ZnO precursor)
- 2-Methoxyethanol (2ME) (Solvent)
- Monoethanolamine (MEA) (Stabilizer)
- Absolute Ethanol (Substrate cleaning)
- Glass substrates
Step-by-Step Methodology:
- Substrate Pre-treatment: Clean glass substrates by sonication in ethanol for 15 minutes to remove organic impurities.
- Sol Preparation: Disperse zinc acetate dihydrate in a mixture of 2-methoxyethanol and monoethanolamine (MEA) at room temperature. Stir the mixture for 2 hours at 50°C. Subsequently, age the solution at room temperature for 24 hours to allow for viscosity adjustment.
- Film Deposition: Deposit the solution onto the pre-cleaned glass substrates via spin coating (e.g., 3000 rpm for 30 seconds). This forms a single layer of the film.
- Drying and Annealing: Dry the coated layer in open air at room temperature. Repeat the coating process to achieve multiple layers (e.g., 3 or 6 layers). Finally, anneal the films at 500°C in air to decompose the zinc acetate into crystalline ZnO nanoparticles.
Photocatalytic Testing with Laser Irradiation:
- Setup: Use a UV laser source (e.g., Argon ion laser tuned to 305 nm). Expand the laser beam to cover the entire coated area of the glass substrate. The power density can be calculated as Total Laser Power / Cross-sectional Area (e.g., 0.25 mW/cm² for a 5 mW beam over a 5 cm diameter) [71].
- Procedure: Immerse the coated substrate in a beaker containing the MB solution (e.g., 25 mL of 14.8 µM). Place the beaker in a dark room with continuous stirring. Before irradiation, take a sample (2 mL) as the zero-time reference.
- Irradiation and Sampling: Expose the substrate to the expanded UV laser beam. Withdraw samples at various time intervals (e.g., 5, 10, 30, 60, 120, 180, 300 min).
- Analysis: Measure the absorbance of the MB solution at 664 nm (monomer) and 612 nm (dimer) using a UV-Vis spectrophotometer to track the degradation of both forms.
Diagram 2: Sol-gel photocatalyst synthesis and testing workflow.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Sol-Gel Photocatalyst Synthesis and Testing
| Reagent/Material | Function/Application | Examples from Literature |
|---|---|---|
| Titanium Alkoxides (e.g., TTIP, Titanium butoxide) | Primary precursor for TiOâ‚‚ synthesis; hydrolyzes to form the metal oxide network. | Used in organic [65] and aqueous [65] sol-gel routes for TiOâ‚‚. |
| Zinc Acetate Dihydrate | Common precursor for the synthesis of ZnO nanoparticles and thin films. | Used in the synthesis of ZnO thin films for MB degradation [71]. |
| Dopant Precursors (e.g., Ag acetate, Fe nitrate, Cerium nitrate) | Introduce dopant ions to modify band gap, reduce charge recombination, and extend light absorption. | Ag NP-doped TiO₂ [65]; Fe₂O₃-doped TiO₂ [72]; CeO₂ in TiO₂ [68]. |
| Solvents (e.g., Ethanol, 2-Methoxyethanol, Isopropanol) | Dissolve precursors and control the viscosity and reaction kinetics of the sol. | Ethanol used in Fe₂O₃-TiO₂ synthesis [72]; 2-Methoxyethanol used for ZnO films [71]. |
| Stabilizers/Chelating Agents (e.g., Monoethanolamine - MEA, Acetic acid) | Control hydrolysis rate, prevent precipitation, and improve sol stability. | MEA used in ZnO synthesis [71]; Acetic acid used in TiOâ‚‚ synthesis [65]. |
| Model Pollutants (e.g., Methylene Blue, 2,4-D, Tetracycline) | Standard compounds for evaluating and benchmarking photocatalytic performance. | Methylene Blue [65] [71]; 2,4-Dichlorophenoxyacetic acid [68] [72]; Tetracycline [69]. |
| Phenol--oxotitanium (2/1) | Phenol--oxotitanium (2/1), CAS:20644-86-4, MF:C12H12O3Ti, MW:252.09 g/mol | Chemical Reagent |
| Benzene, 1-butyl-4-ethyl | Benzene, 1-butyl-4-ethyl, CAS:15181-08-5, MF:C12H18, MW:162.27 g/mol | Chemical Reagent |
Overcoming Synthesis Challenges and Performance Limitations in Photocatalyst Development
The sol-gel method is a cornerstone technique for synthesizing metal oxide photocatalysts, prized for its low-temperature processing, compositional control, and ability to produce high-purity materials [73]. This bottom-up approach involves the transition of a system from a liquid "sol" into a solid "gel" network through hydrolysis and condensation reactions [74]. Despite its advantages, researchers frequently encounter three persistent challenges that can compromise material performance: cracking during drying, phase separation in multi-component systems, and poor crystallinity of the metal oxide phase. These issues are particularly critical in photocatalytic applications where surface area, phase purity, and structural integrity directly impact charge carrier generation, separation, and interfacial reactions [7] [75]. This application note provides a systematic analysis of these challenges within the context of metal oxide photocatalyst synthesis, offering evidence-based protocols and solutions to enhance research reproducibility and material performance.
Understanding and Preventing Cracking
Cracking during the drying stage represents one of the most common defects in sol-gel derived materials. This phenomenon primarily occurs due to the development of capillary stresses within the porous gel network as the solvent evaporates.
Root Causes and Mechanisms
When the liquid phase evaporates from the gel's interconnected pore network, the resulting liquid-vapor menisci generate compressive stresses on the solid matrix. These capillary stresses become particularly severe when pore sizes fall below 20 nm and can catastrophically fracture the gel if uncontrolled [74]. The risk of cracking escalates with higher drying rates, smaller pore sizes, and weaker gel mechanical strength.
Practical Solutions and Protocols
Multiple strategies have been developed to mitigate cracking by controlling stress development during drying:
Controlled Drying Conditions: Implement gradual drying protocols with precisely managed temperature and humidity ramping. Slow evaporation rates reduce stress concentration within the gel network.
Chemical Modification: Employ surfactants or drying control chemical additives (DCCAs) to modify liquid surface tension, thereby reducing capillary pressure [74]. For silica systems, acetic acid catalysis has been shown to reduce defect density and promote more controlled polycondensation, enhancing structural integrity [6].
Microstructural Engineering: Optimize hydrolysis and condensation rates to create more uniform, monodisperse pore sizes that distribute stresses more evenly [74]. In ZnO-SiO2 composites, maintaining an ethanol-to-precursor ratio of 10:1 improves miscibility and prevents premature gelation, leading to more robust networks [6].
Advanced Drying Techniques: For critical applications, utilize supercritical drying (which avoids liquid-vapor interfaces entirely) to produce aerogels with minimal cracking [74] [39]. This method preserves the porous network but requires specialized equipment.
Table 1: Strategies for Preventing Cracking in Sol-Gel Derived Photocatalysts
| Strategy | Mechanism of Action | Application Example | Limitations |
|---|---|---|---|
| Controlled Evaporation | Reduces capillary stress gradients | Gradual drying from 75°C to 120°C for SiO2 [6] | Increases processing time |
| Surface Tension Modification | Lowers capillary pressure | Addition of surfactants to precursor solution [74] | May introduce impurities |
| Aging Treatment | Strengthens gel network | 18-hour aging for SiO2 to strengthen Si-O-Si network [6] | Adds processing step |
| Supercritical Drying | Eliminates liquid-vapor interface | Production of high-surface-area aerogels [74] | Requires specialized equipment |
Avoiding Phase Separation
Phase separation poses a significant challenge in synthesizing multi-component metal oxide photocatalysts, where homogeneous elemental distribution is crucial for creating effective heterojunctions and controlling band structure.
Root Causes and Mechanisms
Phase separation typically results from differences in hydrolysis and condensation rates between precursors, leading to compositional inhomogeneity [5]. In photocatalytic nanocomposites such as ZnO-SiO2, incompatible reaction kinetics can cause preferential precipitation of one phase, creating segregated domains rather than a homogeneous mixed oxide [6]. This segregation diminishes the interfacial contact crucial for efficient charge transfer in photocatalytic systems.
Practical Solutions and Protocols
Achieving molecular-level homogeneity requires strategic control of precursor chemistry and reaction conditions:
Chelating Agents: Utilize complexing ligands (e.g., acetylacetonate, citric acid) to moderate the reactivity of fast-hydrolyzing precursors. This approach equalizes condensation rates between different metal centers [5]. The vanadium oxide acetylacetonate sol-gel method demonstrates how chelation enables better control over film formation [39].
Precursor Selection: Choose precursors with matched hydrolysis kinetics, or modify alkoxide ligands to tune reactivity. For example, in ZnO-SiO2 nanocomposites, zinc acetate dihydrate provides more controlled reaction kinetics compared to nitrate alternatives [6].
Chemical Modification: Partially pre-hydrolyze slower-reacting precursors before adding faster-reacting components. In one-pot syntheses, this method ensures all components reach the gelation point simultaneously [5].
Process Parameter Optimization: Carefully control water content, pH, and temperature to balance reaction rates. Acid-catalyzed conditions often promote more homogeneous mixing through the formation of linear polymers that intertwine during gelation [74].
The successful synthesis of homogeneous ZnO-SiO2 (10:90) nanocomposites, as confirmed by homogeneous elemental distribution in SEM-EDS mapping, demonstrates the effectiveness of these strategies [6].
Improving Crystallinity
The photocatalytic activity of metal oxides depends critically on their crystallinity, as well-defined crystal structures minimize charge carrier recombination and enhance charge separation [75].
Root Causes and Mechanisms
Poor crystallinity in sol-gel derived materials often results from low processing temperatures that are insufficient for complete crystallization, organic residues that inhibit crystal growth, or inappropriate heating rates that cause premature densification [39]. In photocatalytic applications, this leads to high defect densities that act as recombination centers for photogenerated electrons and holes, diminishing photocatalytic efficiency [7] [75].
Practical Solutions and Protocols
Crystallinity can be systematically improved through optimized thermal treatment and precursor management:
Controlled Calcination: Implement multi-stage thermal programs with carefully ramped temperatures. For example, ZnO nanoparticles achieve optimal crystallinity when annealed at 450°C, which effectively removes residual acetate groups while promoting crystal growth without excessive particle aggregation [6].
Dwell Times: Incorporate intermediate temperature holds to facilitate gradual organic removal before crystallization begins. This approach prevents bloating and pore collapse that can occur from rapid volatile release.
Atmosphere Control: Utilize different gas environments during thermal treatment to control defect chemistry. Calcination in a reducing atmosphere can create oxygen vacancies that enhance photocatalytic activity in some metal oxides, while oxidizing conditions ensure complete removal of organic residues [39].
Nucleation Control: Manage precursor chemistry and solution conditions to promote homogeneous nucleation. In ZnO synthesis, using ethanol rather than water as the solvent slows hydroxide ion generation, enabling more controlled growth of crystalline nanorods [6].
Table 2: Thermal Treatment Parameters for Enhanced Crystallinity
| Material | Optimal Annealing Temperature | Dwell Time | Atmosphere | Resulting Crystalline Phase |
|---|---|---|---|---|
| ZnO | 450°C [6] | 1-2 hours | Air | Wurtzite |
| SiO2 | 700-900°C [6] | 2-4 hours | Air | Amorphous (maintained) |
| ZnO-SiO2 Composite | 700-900°C [6] | 2-4 hours | Air | ZnO wurtzite in amorphous SiO2 |
| TiO2 | 400-500°C [39] | 1-3 hours | Air/Controlled | Anatase/Rutile |
Sol-Gel Workflow with Critical Issues and Solutions
Integrated Experimental Protocol: ZnO-SiOâ‚‚ Nanocomposite Synthesis
This protocol exemplifies the application of the above principles to synthesize a photocatalytically relevant ZnO-SiOâ‚‚ nanocomposite system [6].
Materials and Equipment
- Precursors: Tetraethyl orthosilicate (TEOS, ≥99%), zinc acetate dihydrate (Zn(CH₃COO)₂·2H₂O, ≥99%)
- Solvents: Ethanol (anhydrous), deionized water
- Catalysts: Acetic acid (glacial), sodium hydroxide (1M solution)
- Equipment: Magnetic stirrer with temperature control, drying oven, muffle furnace, pH meter, airtight containers
Step-by-Step Procedure
Silica Sol Preparation (Day 1)
- Prepare a homogeneous solution with TEOS:EtOH:Hâ‚‚O molar ratio of 1:10:4
- Add acetic acid (0.05 mol per mol TEOS) as catalyst under continuous stirring at 300 rpm
- Maintain reaction at 25°C for 3 hours until a clear sol forms
- Transfer to sealed container and initiate gelation at 75°C for 18 hours
Zinc Oxide Incorporation (Day 2)
- Dissolve zinc acetate dihydrate (10g in 100mL ethanol) with stirring until fully dissolved
- Slowly add the zinc acetate solution to the aged silica gel under vigorous stirring
- Adjust pH to neutral using 1M NaOH solution to initiate ZnO formation
- Continue stirring for 4 hours to ensure homogeneous distribution
Aging and Drying (Day 2-3)
- Age the composite gel for 24 hours at room temperature to strengthen the network
- Transfer to drying oven and implement gradual temperature ramp: 75°C (2h) → 100°C (2h) → 120°C (4h)
- Cool slowly to room temperature in oven to minimize thermal stress
Thermal Treatment (Day 4)
- Place dried gel in muffle furnace with air atmosphere
- Implement controlled calcination program:
- Ramp 2°C/min to 450°C, dwell 2h (for ZnO crystallization)
- For higher temperature treatment: ramp 3°C/min to 700-900°C, dwell 4h
- Allow furnace to cool naturally to room temperature
Characterization and Validation
- XRD: Confirm wurtzite ZnO phase and amorphous SiOâ‚‚ matrix
- FT-IR: Verify Zn-O-Si bond formation at ~950 cmâ»Â¹ in addition to Si-O-Si (1100 cmâ»Â¹) and Zn-O (450 cmâ»Â¹) vibrations
- SEM-EDS: Validate homogeneous elemental distribution of Zn, Si, and O
- BET Surface Area: Ensure maintenance of porous structure (>200 m²/g)
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Sol-Gel Synthesis of Metal Oxide Photocatalysts
| Reagent | Function | Example Application | Considerations |
|---|---|---|---|
| Metal Alkoxides | Molecular precursors for oxide network | TEOS for SiOâ‚‚, Ti alkoxides for TiOâ‚‚ | Moisture-sensitive; require anhydrous handling |
| Chelating Agents | Modulate precursor reactivity | Acetylacetone for slower hydrolysis | Can affect final stoichiometry |
| Surfactants | Control pore structure, reduce cracking | CTAB for mesoporous materials | Requires calcination for removal |
| Acid/Base Catalysts | Control hydrolysis/condensation rates | Acetic acid for SiOâ‚‚, NaOH for ZnO | pH critically affects gel structure |
| Solvent Systems | Dissolve precursors, control reaction | Ethanol for ZnO nanorods [6] | Polarity affects nucleation kinetics |
| Chloro(isopropyl)silane | Chloro(isopropyl)silane, MF:C3H7ClSi, MW:106.62 g/mol | Chemical Reagent | Bench Chemicals |
| Lanthanum--nickel (2/7) | Lanthanum--nickel (2/7), CAS:12532-78-4, MF:La2Ni7, MW:688.66 g/mol | Chemical Reagent | Bench Chemicals |
The successful application of sol-gel synthesized metal oxides in photocatalysis hinges on overcoming the interrelated challenges of cracking, phase separation, and poor crystallinity. By implementing the systematic approaches outlined in this application note—including controlled drying protocols, precursor engineering through chelation, and optimized thermal treatments—researchers can significantly enhance the structural integrity, phase homogeneity, and crystalline quality of their photocatalyst materials. The provided integrated protocol for ZnO-SiO₂ nanocomposites demonstrates how these principles can be applied in practice to fabricate advanced photocatalytic systems with improved performance. As sol-gel science continues to evolve, coupling these fundamental strategies with emerging approaches such as machine-learning-assisted optimization and green chemistry principles will further advance the field of metal oxide photocatalysis [3].
In photocatalytic applications, from hydrogen production via water splitting to the degradation of environmental pollutants, the rapid recombination of photogenerated electron-hole pairs represents a fundamental bottleneck limiting efficiency. [76] [77] When a semiconductor absorbs light, electrons are excited from the valence band (VB) to the conduction band (CB), creating holes in the VB. These charge carriers must migrate to the surface to drive redox reactions before they recombine and dissipate their energy as heat or light. [76] This application note details two paramount strategies—cocatalyst integration and heterostructure engineering—to suppress this recombination, with a specific focus on metal oxide photocatalysts synthesized via the sol-gel method. The content is structured to provide researchers with both the theoretical foundation and practical protocols necessary to enhance the performance of photocatalytic systems.
Theoretical Background: The Recombination Challenge
The photocatalytic process involves several critical steps: (1) light absorption and carrier generation, (2) charge separation and migration, and (3) surface redox reactions. [78] The efficiency of the entire process is often governed by the second step, as the lifetimes of the photogenerated carriers are typically short.
A critical electronic consideration is the configuration of the metal centers in transition metal oxides. Recent studies have established a direct link between the electronic configuration and carrier lifetime. [79] Oxides with d0 (e.g., TiO2) or d10 configurations can generate long-lived charge carriers, whereas open d-shell oxides (e.g., Fe2O3, Co3O4) often exhibit sub-picosecond relaxation via metal-centered ligand field states, which act as rapid recombination centers. [79] This insight is crucial for selecting the primary semiconductor material in a photocatalytic system.
Strategy 1: Cocatalyst Engineering
The Role and Mechanism of Cocatalysts
A cocatalyst is an additional component, typically loaded in small amounts onto the primary semiconductor, which synergistically enhances photocatalytic performance without being the main light absorber. [78] Cocatalysts function through several interconnected mechanisms:
- Providing Active Reaction Sites: They offer specific surface sites that lower the activation energy for surface redox reactions, such as proton reduction for H2 evolution. [78]
- Promoting Charge Separation: Cocatalysts often act as electron sinks, selectively extracting photogenerated electrons from the semiconductor bulk. This physical separation of electrons and holes drastically reduces the probability of their recombination. [78]
- Improving Reaction Kinetics: By facilitating the adsorption of reactants and desorption of products, cocatalysts accelerate the surface reaction rates, thereby preventing the accumulation of charge carriers and further suppressing recombination. [78]
Classification of Cocatalysts
cocatalyst materials for photocatalytic hydrogen evolution [78]
| Cocatalyst Category | Representative Materials | Key Function & Characteristics |
|---|---|---|
| Noble Metals | Pt, Pd, Au, Ag, Ru | Highly active; act as electron sinks; but high cost and low abundance. |
| Metal Oxides | NiO, CuO | Earth-abundant; can form p-n junctions with the main semiconductor. |
| Transition Metal Dichalcogenides | MoS2, WS2 | Provide abundant edge sites for H2 evolution; tunable properties. |
| Metal Phosphides | Ni2P, CoP | High conductivity and excellent catalytic activity. |
| Metal Carbides/Borides | Mo2C, Ti3C2 (MXenes) | Good electrical conductivity and stability. |
| Carbon-Based | Graphene, Carbon Nanotubes | Excellent electron acceptors and transporters; high surface area. |
| Single-Atom Cocatalysts | Pt1, Pd1 on supports | Maximizes atom utilization; high activity and unique electronic structures. |
Protocol: Loading a Noble Metal Cocatalyst via Photodeposition
This protocol details the deposition of platinum (Pt) nanoparticles onto sol-gel-synthesized TiO2 for enhanced hydrogen evolution.
Research Reagent Solutions:
- Primary Photocatalyst: 500 mg of sol-gel synthesized TiO2 powder (anatase phase).
- Cocatalyst Precursor: Chloroplatinic acid (H2PtCl6) solution (1 mg/mL in deionized water).
- Sacrificial Agent: Methanol (ACS reagent grade, ≥99.9%).
- Solvent: Deionized water (18.2 MΩ·cm resistivity).
Procedure:
- Slurry Preparation: Disperse the 500 mg of TiO2 powder in 200 mL of a 25% v/v aqueous methanol solution in a quartz photoreactor. The methanol acts as a sacrificial electron donor.
- Precursor Addition: Add a calculated volume of the H2PtCl6 stock solution to the slurry to achieve a nominal Pt loading of 1 wt% relative to TiO2. Stir continuously in the dark for 30 minutes to ensure homogeneous adsorption of the precursor onto the semiconductor surface.
- Photodeposition: Irradiate the stirred slurry under a 300 W Xenon lamp (or equivalent UV light source) for 1 hour. During irradiation, photogenerated electrons from the TiO2 conduction band reduce the Pt4+ ions to metallic Pt (Pt0), forming nanoparticles on the TiO2 surface.
- Post-processing: After irradiation, recover the Pt/TiO2 powder by centrifugation. Wash the powder three times with deionized water to remove any ions or residual organics.
- Drying: Dry the final product in an oven at 80°C for 12 hours before characterization and photocatalytic testing.
Strategy 2: Heterostructure Engineering
Principles and Types of Heterostructures
Constructing a heterojunction between two different semiconductors is a powerful strategy to create a built-in electric field that drives the spatial separation of electrons and holes. [76] The key is to align the band structures of the two materials appropriately. The main types of heterostructures include:
- Type-II Heterojunction: The band edges are staggered such that electrons migrate to one semiconductor's CB and holes to the other's VB, achieving highly effective charge separation. [76]
- p-n Heterojunction: Formed between a p-type (e.g., Cu2O) and an n-type (e.g., ZnO) semiconductor. The internal electric field in the space charge region drives electrons and holes in opposite directions, suppressing recombination. [76]
Protocol: Constructing a p-n Heterojunction via Sol-Gel Synthesis
This protocol outlines the synthesis of a p-NiO/n-ZnO heterostructure, where the p-n junction enhances charge separation.
Diagram 1: Sol-gel synthesis workflow for heterostructure
Research Reagent Solutions:
- n-type Precursor: Zinc acetate dihydrate (Zn(CH3COO)2·2H2O).
- p-type Precursor: Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O).
- Sol-Gel Agents: Oxalic acid dihydrate ((COOH)2·2H2O) as a chelating agent.
- Solvent: Ethanol (Absolute, ≥99.8%).
- Target Composition: Zn:Ni molar ratio of 9:1.
Procedure:
- Solution A (Zn-precursor): Dissolve zinc acetate dihydrate in 50 mL of ethanol. Stir magnetically for 30 minutes at 50°C until fully dissolved. [17]
- Solution B (Ni-precursor & Gelation Agent): Dissolve nickel nitrate and oxalic acid in 25 mL of ethanol. Stir at room temperature until a clear solution is obtained. [17]
- Combination and Gelation: Slowly add Solution B to Solution A under vigorous stirring. Continue stirring the combined solution at 70°C until a viscous gel forms. [17]
- Aging and Drying: Age the gel for 12 hours at room temperature, then transfer it to an oven to dry at 80°C overnight. [17]
- Calcination: Calcine the dried powder in a muffle furnace at 500°C for 2-4 hours in air. This step decomposes the precursors and oxalates, leading to the crystallization of the NiO/ZnO composite. [17] [18]
Performance Comparison and Data Presentation
The effectiveness of cocatalysts and heterostructures is quantitatively demonstrated by their impact on photocatalytic reaction rates and carrier lifetimes. The table below summarizes performance data for various modified photocatalysts.
Performance of engineered photocatalysts [76] [17] [18]
| Photocatalyst System | Modification Strategy | Application | Performance Metric | Key Result |
|---|---|---|---|---|
| TiO2 | Pt cocatalyst | H2 Evolution | H2 Evolution Rate | >50x increase vs. bare TiO2 [78] |
| ZnO | Solvent optimization (Ethanol) | Dye Degradation | Degradation Efficiency | 98% in a short duration [17] |
| BaTi5O11 | Solvent optimization (PEG-200) | Dye Degradation | Degradation Time & Surface Area | Complete degradation in 30 min; BET: 9.78 m²/g [19] |
| 90TiO2-10Fe2O3/PVP | Composite/Heterostructure | Antibiotic Degradation | Degradation Efficiency | Superior to commercial TiO2 P25 [18] |
| d0 / d10 TMOs (e.g., TiO2) | Intrinsic Electronic Structure | General Photocatalysis | Carrier Lifetime | Long-lived, high quantum yields [79] |
Suppressing electron-hole recombination is a central challenge in photocatalysis. As detailed in this application note, the strategic engineering of photocatalysts through the incorporation of cocatalysts and the construction of heterostructures provides a powerful, synergistic path forward. Cocatalysts function primarily as efficient electron sinks and reactive sites, while heterostructures create intrinsic electric fields that physically separate charge carriers. The provided protocols for sol-gel synthesis and modification offer a practical roadmap for researchers to implement these strategies. The continued refinement of these approaches, guided by insights into electronic configuration and interfacial charge dynamics, is essential for advancing the field of solar energy conversion.
The sol-gel method has emerged as a powerful and versatile technique for the synthesis of metal oxide photocatalysts, prized for its low-temperature processing, excellent compositional control, and ability to produce homogeneous materials with high specific surface areas [9] [3]. A significant limitation of many wide-bandgap metal oxides, such as TiO₂ and ZnO, is their primary activity under ultraviolet light, which constitutes only a small fraction of the solar spectrum [9] [80]. This constraint has driven extensive research into strategies for enhancing their responsiveness to visible light. This Application Note details three principal advanced strategies—doping, sensitization, and defect engineering—within the context of sol-gel synthesis, providing structured experimental protocols and data to guide researchers in developing efficient visible-light-driven photocatalysts for environmental and energy applications.
Table 1: Comparison of Key Strategies for Enhancing Visible Light Absorption
| Strategy | Mechanism of Action | Key Materials/Examples | Sol-Gel Synthesis Advantages | Primary Challenges |
|---|---|---|---|---|
| Elemental Doping | Introduces new energy levels within the bandgap, narrowing the effective bandgap energy [80]. | Se-doped TiOâ‚‚ [80], Fe-doped TiOâ‚‚ [9], S-doped ZnS [81]. | Molecular-level precursor mixing ensures homogeneous dopant distribution [9] [3]. | Dopant segregation or formation of secondary phases; possible introduction of charge recombination centers [3]. |
| Defect Engineering | Creates vacancies (e.g., oxygen, zinc, sulfur) that generate mid-gap states, extending light absorption and trapping charge carriers to reduce recombination [82] [81]. | α-Fe₂O₃ with oxygen vacancies [82], ZnS with S/Zn vacancies [81]. | Calcination conditions (temperature, atmosphere) can be precisely controlled to tailor defect density and type [82]. | Differentiating between beneficial and detrimental defect types; achieving reproducible defect concentrations [82] [81]. |
| Sensitization & Composite Formation | A sensitizer (e.g., graphene, dye) with a narrow bandgap absorbs visible light and injects electrons into the metal oxide conduction band [83] [84]. | TiOâ‚‚/graphene hybrids [84], ZnO-SiOâ‚‚ composites [6]. | Facilitates the creation of intimate interfaces and anchoring of sensitizers onto the oxide network [84] [6]. | Ensuring efficient charge transfer across the interface; long-term stability of the sensitizer [83]. |
The experimental workflow for developing these enhanced photocatalysts via sol-gel synthesis involves several key stages, from precursor preparation to final performance testing.
Detailed Experimental Protocols
Protocol 1: Sol-Gel Synthesis of Se-Doped TiOâ‚‚ Nanoparticles
This protocol outlines the synthesis of Se-doped TiOâ‚‚ with a significantly narrowed bandgap for visible-light-driven degradation of organic dyes like Rhodamine B (RhB) [80].
3.1.1 Research Reagent Solutions Table 2: Key Reagents for Se-Doped TiOâ‚‚ Synthesis
Reagent Function Specification / Note Tetrabutyl Titanate (Câ‚₆H₃₆Oâ‚„Ti) TiOâ‚‚ precursor Use as received, analytical grade Selenium Dioxide (SeOâ‚‚) Dopant precursor (Source of Seâ´âº) Sublimes at 315-317°C; low calcination temp is critical [80] Absolute Ethanol (Câ‚‚Hâ‚…OH) Solvent Anhydrous Nitric Acid (HNO₃) Catalyst for hydrolysis & peptization Dilute solution Rhodamine B (RhB) Model pollutant for activity testing 7.5 mg·Lâ»Â¹ in aqueous solution 3.1.2 Step-by-Step Procedure
- Solution A: Mix 20 mL of tetrabutyl titanate with 40 mL of absolute ethanol under vigorous stirring until a transparent solution is obtained. Add nitric acid dropwise to adjust the pH to ~3-4, which catalyzes hydrolysis and prevents premature precipitation.
- Solution B: Dissolve SeOâ‚‚ in 20 mL of absolute ethanol. The amount of SeOâ‚‚ is based on the target atomic concentration of Se in Tiâ‚â‚‹â‚“Seâ‚“Oâ‚‚ (e.g., 0, 5, 10, 15 at.%) [80].
- Mixing and Gelation: Add Solution B dropwise into Solution A under continuous violent stirring. Continue stirring for 2 hours after complete addition until a uniform sol is formed.
- Aging and Drying: Allow the sol to stand at room temperature for 16 hours to form a wet gel. Dry the gel in an oven at 120°C for 6-12 hours to obtain a dry xerogel.
- Calcination: Calcine the xerogel in a muffle furnace at 300°C for 3 hours. The low calcination temperature is crucial to prevent the sublimation of SeO₂ and achieve high doping concentrations [80].
- Characterization and Testing: Grind the final product into a fine powder for characterization and photocatalytic testing.
3.1.3 Performance Data Table 3: Characterization and Performance of Se-Doped TiOâ‚‚ [80]
Sample (Designated) Measured Se (at.%) Crystal Size (nm) Band Gap (eV) RhB Degradation Efficiency Pure TiOâ‚‚ 0 12.4 3.20 Baseline (Low) TSe5 3.8 15.1 2.81 Enhanced TSe10 8.5 11.2 2.45 Significantly Enhanced TSe15 13.6 9.8 2.17 Highest
Protocol 2: Green Synthesis and Defect Engineering of α-Fe₂O₃ Nanoparticles
This protocol uses a bio-assisted sol-gel method with Maytenus rigida extract, where calcination controls the phase and defect density, enhancing visible-light photocatalysis [82].
3.2.1 Research Reagent Solutions Table 4: Key Reagents for α-Fe₂O₃ Synthesis
Reagent Function Specification / Note Iron(III) Nitrate Nonahydrate (Fe(NO₃)₃·9H₂O) Fe₂O₃ precursor ≥99% Maytenus rigida Hydroethanolic Extract Bio-template, chelating & reducing agent Prepared as per established protocol [82] Hexamethyldisiloxane (HDMSO) Solvent ≥99% Methylene Blue (MB) Model pollutant for activity testing ≥99% 3.2.2 Step-by-Step Procedure
- Complexation: Dissolve iron nitrate nonahydrate in HDMSO. Add the Maytenus rigida extract dropwise to this solution under constant stirring. The phytochemicals (tannins, flavonoids) complex with Fe³⺠ions.
- Gelation and Aging: Stir the mixture for 4 hours at 60°C until a viscous gel forms. Age the gel for 24 hours at room temperature.
- Drying: Dry the gel at 100°C for 12 hours to obtain a xerogel precursor.
- Calcination (Defect Engineering): Calcine portions of the xerogel at different temperatures (e.g., 400°C, 500°C, 600°C) for 2 hours. This step is critical for driving the phase transition from a mixed Fe₃O₄/α-Fe₂O₃ system to pure α-Fe₂O₃ and for introducing oxygen vacancies that enhance photocatalytic activity [82].
- Characterization and Testing: The magnetic properties of the nanoparticles allow for easy recovery using an external magnet after water treatment cycles [82].
3.2.3 Performance Data Calcination at 500°C was found to produce pure α-Fe₂O₃ with an optimal defect density, leading to a 95.6% degradation efficiency of Methylene Blue under visible light in 180 minutes. The enhanced activity is attributed to defect-mediated charge separation [82].
Protocol 3: Synthesis of TiOâ‚‚/Graphene Hybrids for Sensing and Abatement
This protocol describes the one-pot sol-gel synthesis of a hybrid material with dual functionality for NOâ‚‚ sensing and photocatalytic abatement [84].
3.3.1 Research Reagent Solutions The synthesis uses standard TiOâ‚‚ precursors (e.g., titanium alkoxides) with graphene added directly to the reaction vessel prior to initiating the sol-gel reaction [84].
3.3.2 Step-by-Step Procedure
- Dispersion: Disperse graphene in the solvent (e.g., ethanol) using sonication.
- Sol-Gel with Graphene: Add the TiOâ‚‚ precursors to the graphene dispersion to initiate the hydrolytic sol-gel reaction. This method (denoted as GTiO2S) creates an intimate mixture, superior to simply mixing graphene with pre-formed TiOâ‚‚ nanoparticles [84].
- Gelation and Aging: Allow the mixture to gel and age for 12-24 hours.
- Annealing: Anneal the resulting material at 450-500°C in an inert atmosphere to crystallize the TiO₂ without degrading the graphene.
- Testing: The material can be fabricated into a conductometric sensor for NOâ‚‚ sensing and simultaneously tested for its ability to photocatalytically degrade NOâ‚“ under simulated sunlight [84].
3.3.3 Performance Data The GTiO2S hybrid showed a sensor response to 1750 ppb NOâ‚‚ that was approximately double under UV irradiation compared to the response in the dark, with a detection limit of ~50 ppb. It also demonstrated high activity for the photocatalytic degradation of NOâ‚“ [84].
The mechanism of enhanced photocatalysis in these engineered materials relies on the efficient generation and separation of charge carriers upon light absorption.
The strategic enhancement of sol-gel-synthesized metal oxide photocatalysts through doping, defect engineering, and sensitization is a cornerstone of modern photocatalysis research. The protocols detailed herein provide a reproducible framework for achieving high-performance materials capable of operating efficiently under visible light. The integration of these strategies, such as creating doped and defect-rich materials or intimately mixed heterostructures, represents the future direction for developing advanced photocatalytic systems for environmental remediation, renewable energy generation, and sensing applications.
The sol-gel method for synthesizing metal oxide photocatalysts presents a powerful pathway for designing materials with precise control over structural and photocatalytic properties. However, transitioning from laboratory-scale synthesis to industrial-scale production introduces significant challenges, including maintaining material homogeneity, achieving consistent product quality, and managing energy consumption. Microwave-assisted synthesis has emerged as a transformative approach that addresses these scaling challenges through rapid, uniform heating mechanisms that substantially reduce processing time and energy usage compared to conventional thermal methods [85] [86]. This application note systematically examines the scaling challenges in conventional sol-gel processes and provides detailed protocols for implementing microwave-assisted approaches with optimized reactor design, enabling researchers to overcome critical barriers in photocatalyst production.
Scaling-Up Challenges in Conventional Sol-Gel Synthesis
The conventional sol-gel synthesis of metal oxide photocatalysts faces multiple challenges when transitioning from laboratory to industrial scale. The table below summarizes the primary constraints and their implications for photocatalyst quality and production efficiency.
Table 1: Key Challenges in Scaling-Up Conventional Sol-Gel Synthesis of Metal Oxide Photocatalysts
| Challenge Category | Specific Limitations | Impact on Photocatalyst Production |
|---|---|---|
| Thermal Management | Heterogeneous heating via conduction/convection [87] | Inconsistent crystal growth and phase distribution |
| Extended processing times (hours to days) [87] [88] | Limited throughput and high energy consumption | |
| Process Control | Difficulties in maintaining uniform gelation [87] | Variable porosity and surface area characteristics |
| Sensitivity to operational parameters [89] | Batch-to-batch inconsistencies | |
| Material Quality | Particle agglomeration [89] | Reduced surface area and photocatalytic activity |
| Cracking and shrinkage during drying [89] | Structural defects impacting performance | |
| Economic Factors | High energy consumption [86] | Increased production costs |
| Requirement for toxic chemicals/epoxides [88] | Additional safety and environmental concerns |
These challenges become particularly pronounced in the synthesis of transition metal oxide photocatalysts such as iron-based aerogels and titanium dioxide variants, where precise control over crystallinity, phase composition, and surface properties directly determines photocatalytic performance in applications such as organic pollutant degradation and energy conversion [87] [90] [88].
Microwave-Assisted Solutions: Principles and Advantages
Microwave-assisted synthesis represents a paradigm shift in sol-gel processing by utilizing electromagnetic energy (0.3-300 GHz) to generate heat volumetrically within the reaction mixture rather than relying on surface-to-core thermal transfer [86]. This fundamental difference in heating mechanism addresses many of the inherent limitations of conventional scale-up approaches.
Table 2: Comparative Analysis of Conventional vs. Microwave-Assisted Sol-Gel Synthesis
| Parameter | Conventional Heating | Microwave-Assisted | Impact on Scaling Potential |
|---|---|---|---|
| Heating Mechanism | Conduction/Convection (surface-to-core) [86] | Volumetric (molecular interaction with radiation) [86] | Eliminates thermal gradients in large volumes |
| Reaction Time | Hours to days [87] [88] | Minutes to hours [91] [88] | Significantly improved production throughput |
| Energy Efficiency | Low (heat loss to surroundings) [86] | High (selective energy absorption) [86] | Reduced operational costs at scale |
| Temperature Control | Slow response, gradients [87] | Rapid, precise regulation [88] | Enhanced reproducibility across batches |
| Product Quality | Inconsistent morphology [89] | Uniform crystallinity and particle size [91] | Reliable photocatalyst performance |
| Environmental Impact | Often requires toxic chemicals [88] | Reduced waste generation [86] | More sustainable production process |
The advantages of microwave-assisted approaches extend beyond accelerated reaction kinetics to encompass improved product characteristics. Studies demonstrate that microwave-synthesized yttrium-doped TiOâ‚‚ systems exhibit enhanced photo-oxidation efficiency for pollutants like carbamazepine, attributed to superior charge transfer characteristics, increased surface area, and optimal crystallite size [91]. Similarly, microwave-assisted synthesis enables precise control over the structural properties of iron-based aerogels, allowing tailored micro-meso-macroporosity for electrochemical applications [88].
Reactor Design Considerations for Scaling-Up
Reactor design represents a critical factor in successful scale-up of microwave-assisted sol-gel synthesis. The interaction between the electromagnetic field and reaction vessel geometry directly influences heating homogeneity and ultimately determines the structural properties of the synthesized photocatalysts.
Vessel Geometry and Configuration
Experimental investigations demonstrate that vessel shape profoundly affects the microwave heating profile and the resulting material characteristics. Research on iron-based aerogel synthesis reveals that "a wide vessel is preferable to a tall and narrow one since the heating process is more homogeneous in the former and the sol-gel and cross-linking reactions take place earlier, which improves the mechanical properties of the final nanomaterial" [87]. The schematic workflow below illustrates the critical parameters in reactor design for scalable microwave-assisted synthesis:
Schematic Workflow of Reactor Design Parameters and Their Impact on Final Material Properties
Scaling Configuration Strategies
Three primary approaches have been evaluated for scaling up microwave-assisted sol-gel synthesis:
- Single Vessel Scaling: Increasing precursor volume in appropriately shaped vessels [87]
- Multiple Vessel Systems: Simultaneous processing of multiple containers in multimode cavities [87]
- Continuous Flow Reactors: Emerging approach for industrial-scale production [86]
Experimental data indicates that "the shape and size of the vessel can be determinant in the interaction with microwaves and, thus, in the heating process, influencing the sol-gel reactions and the characteristics and homogeneity of the obtained nanomaterials" [87]. For mass production, the interaction of reagents with the microwave field must be carefully considered, as this depends not only on their chemical nature but also on their volume, shape, and arrangement inside the cavity [87].
Detailed Experimental Protocols
Protocol 1: Microwave-Assisted Synthesis of Iron-Based Aerogels
This protocol outlines the optimized procedure for synthesizing iron-based aerogels with controlled porosity and magnetic properties [88].
Research Reagent Solutions
Table 3: Essential Reagents for Iron-Based Aerogel Synthesis
| Reagent | Specifications | Function in Synthesis |
|---|---|---|
| Iron(II) chloride (FeClâ‚‚) | Sigma-Aldrich, 98% purity [88] | Metallic precursor providing iron ions |
| Sodium carbonate (Na₂CO₃) | Indspec, 99% purity [88] | Alkalinity agent for gelation control |
| Glyoxylic acid (C₂H₂O₃) | Sigma-Aldrich, 98% purity [88] | Reducing agent for sol-gel reaction |
| Deionized water | N/A | Solvent for precursor solutions |
Step-by-Step Procedure
Precursor Solution Preparation:
- Prepare reducing solution (Solution R) by dissolving 1.5 g sodium carbonate and 250 mg glyoxylic acid in 250 mL deionized water [88].
- Prepare metallic solution (Solution M) by dissolving FeClâ‚‚ in deionized water with a concentration of 2 mg/mL [88].
- Mix solutions R and M in volumetric ratios ranging from 4:1 to 1:4 (R:M) depending on desired porosity characteristics [88].
Microwave Processing:
- Transfer 20 mL of precursor solution to microwave-transparent Teflon vessels [88].
- Place vessels in multimode microwave reactor (e.g., Milestone ETHOS 1) equipped with rotation system for homogeneous exposure [88].
- Maintain temperature constant at 68°C for 1-8 hours, depending on target structural properties [88].
- Monitor temperature using thermocouple inserted directly into one vessel.
Post-Processing:
- Remove excess water after reaction completion.
- Wash precipitate by centrifugation (3500 rpm, 5 min/cycle, 5 cycles) to eliminate unreacted products [88].
- Freeze samples with liquid nitrogen.
- Dry in freeze dryer for 24 hours to obtain final FeA.
Characterization and Expected Outcomes
- Textural Properties: BET surface area analysis reveals tailorable micro-meso-macroporosity based on R:M ratio [88].
- Morphological Control: Cluster- or flake-type structures achievable through time and ratio optimization [88].
- Magnetic Properties: Tunable iron(II) content enables modulation from highly magnetic to non-magnetic materials [88].
Protocol 2: Microwave-Assisted Hydrothermal Synthesis of Yttrium-Doped TiOâ‚‚
This protocol describes the comparative synthesis of yttrium-doped TiOâ‚‚ photocatalysts using microwave-assisted versus conventional hydrothermal methods [91].
Research Reagent Solutions
Table 4: Essential Reagents for Yttrium-Doped TiOâ‚‚ Synthesis
| Reagent | Specifications | Function in Synthesis |
|---|---|---|
| Titanium(IV) chloride (TiClâ‚„) | Merck, 97% purity [91] | Primary titanium precursor |
| Yttrium(III) chloride hexahydrate (YCl₃·6H₂O) | Merck, 99% purity [91] | Yttrium doping source |
| Urea (CHâ‚„Nâ‚‚O) | Merck, p.a. grade [91] | Hydrolysis control agent |
| Deionized water | N/A | Reaction solvent |
Step-by-Step Procedure
Precursor Preparation:
- Prepare 1 wt% TiClâ‚„ solution in distilled water using ice-water bath to control hydrolysis [91].
- Transfer 100 cm³ TiCl₄ solution to reactor vessel.
- Add 1 g urea with continuous stirring for 15 minutes.
Microwave Hydrothermal Treatment:
Yttrium Doping:
- Dissolve 50 mg YCl₃·6H₂O (for 1 wt% yttrium) and 100 mg urea in 100 cm³ water [91].
- Prepare suspension of pre-synthesized TiO₂ in water (1 g TiO₂ in 100 cm³).
- Combine TiOâ‚‚ suspension with yttrium precursor solution, stir 30 minutes.
- Apply microwave hydrothermal treatment: T = 200°C, t = 5 minutes, P = 300W [91].
- Cool, wash, and dry as in previous steps.
Conventional Hydrothermal Comparison:
- For comparative studies, perform conventional hydrothermal treatment at T = 200°C for 12 hours [91].
Characterization and Expected Outcomes
- Structural Properties: XRD confirms anatase tetragonal structure; successful yttrium incorporation more effective in microwave approach [91].
- Photocatalytic Performance: Microwave-synthesized TiOâ‚‚-Y demonstrates enhanced photo-oxidation efficiency for carbamazepine under UV-LED light [91].
- XPS Analysis: Reveals significant difference in yttrium content between microwave and conventional methods [91].
Performance Evaluation and Comparative Analysis
The implementation of optimized microwave-assisted protocols with appropriate reactor design yields significant improvements in photocatalyst performance and characteristics.
Table 5: Comparative Performance of Microwave vs. Conventionally Synthesized Photocatalysts
| Photocatalyst System | Synthesis Method | Key Performance Metrics | Reference |
|---|---|---|---|
| Yttrium-doped TiOâ‚‚ | Microwave-assisted hydrothermal | Enhanced CBZ photo-oxidation efficiency; Improved absorption and charge transfer | [91] |
| Conventional hydrothermal | Lower photocatalytic efficiency; Limited yttrium incorporation | [91] | |
| Iron-based aerogels | Microwave-assisted sol-gel | Tailorable porosity; Controlled magnetic properties; Cluster/flake morphology control | [88] |
| Conventional sol-gel | Long processing times; Toxic chemicals requirement; Limited crystallinity control | [88] | |
| Transition metal aerogels | Scaled microwave approach | Homogeneous structure in wide vessels; Preserved textural properties | [87] |
| Unoptimized microwave | Heterogeneous heating in narrow vessels; Inconsistent morphology | [87] |
The comparative analysis demonstrates that microwave-synthesized photocatalysts consistently outperform conventionally prepared materials in critical performance metrics, including photocatalytic activity, structural control, and phase purity, while simultaneously reducing processing times from days to hours or minutes [91] [88].
Microwave-assisted synthesis, when coupled with optimized reactor design, presents a robust solution to the scaling challenges inherent in conventional sol-gel processes for metal oxide photocatalyst production. The integration of appropriate vessel geometry, controlled power delivery, and optimized reaction parameters enables the transition from laboratory-scale synthesis to industrially relevant production while maintaining precise control over critical material properties.
Successful implementation requires careful attention to:
- Selection of wide, shallow vessel geometries over tall, narrow configurations [87]
- Optimization of power-to-volume ratios to ensure homogeneous heating [87]
- Consideration of multimode systems with rotation capability for batch processing [88]
- Adaptation of reaction times and temperatures to specific photocatalyst systems [91] [88]
The protocols and design principles outlined in this application note provide a foundation for researchers to overcome traditional scaling limitations and advance the development of high-performance photocatalysts for environmental remediation and energy applications. As microwave technology continues to evolve, further innovations in continuous flow systems and intelligent process control promise to enhance the scalability and sustainability of metal oxide photocatalyst manufacturing.
The sol-gel method has emerged as a powerful and versatile technique for the synthesis of metal oxide nanostructures, particularly for photocatalytic applications. This wet-chemical approach enables precise control over composition, morphology, and textural properties at the molecular level, offering distinct advantages for fabricating materials with tailored functionalities [3] [9]. A critical challenge persists, however, in the thermal treatment phase required to transform the amorphous gel into a crystalline metal oxide. This process typically triggers a fundamental trade-off: as crystallinity improves with increasing temperature, the specific surface area (SSA) often dramatically decreases due to particle sintering and agglomeration [92] [93].
This Application Note addresses this central conflict by providing detailed protocols and data-driven strategies to optimize thermal treatments for sol-gel-derived metal oxides. The ability to balance high crystallinity with preserved surface area is paramount for applications such as photocatalysis, gas sensing, and energy conversion, where both efficient charge transport (facilitated by good crystallinity) and abundant reactive sites (provided by high SSA) are essential for peak performance [6] [93].
Key Challenges and Fundamental Relationships
The inverse relationship between crystallinity and surface area during annealing stems from several intrinsic material behaviors:
- Particle Sintering and Agglomeration: Elevated temperatures increase atomic mobility, causing nanoparticles to coalesce and form larger, lower-surface-area aggregates [92].
- Pore Collapse and Densification: The gel's intricate porous network, responsible for its high SSA, becomes unstable and collapses during heating, leading to significant shrinkage and densification [94].
- Phase Transformations: In materials like TiOâ‚‚, high temperatures can induce a phase transition from the high-surface-area anatase phase to the more stable but lower-surface-area rutile phase, further reducing the active surface [95] [92].
The thermal environment—including atmosphere, gas flow, and pressure—profoundly influences these processes. For instance, studies on high-surface-area anatase TiO₂ have demonstrated that thermal treatments in vacuum can preserve surface area up to 450°C, while static calcination in air leads to a significant and less reproducible reduction in SSA [92].
Quantitative Data: Thermal Treatment Impact on Material Properties
The following tables synthesize experimental data from recent studies, illustrating the effects of different thermal parameters on key material characteristics.
Table 1: Impact of Heat Treatment Strategy on ZnO Nanocrystals
| Sample ID | Heat Treatment Process | Crystallite Size (nm) | Specific Surface Area (m²/g) | Key Findings |
|---|---|---|---|---|
| ZnO-650/200 | One-step: 650°C for 200 min | 42.13 | 29.97 | Baseline for comparison [93] |
| ZnO-650/400 | One-step: 650°C for 400 min | 42.40 | 17.61 | Longer duration increased sintering, reducing SSA by ~41% [93] |
| ZnO-300/100–650/200 | Stepwise: 300°C for 100 min, then 650°C for 200 min | 41.40 | 29.35 | Optimal balance: Excellent crystallinity with minimal SSA loss [93] |
Table 2: Effect of Thermal Environment on Anatase TiOâ‚‚
| Treatment Condition | Temperature | Impact on Specific Surface Area | Key Observations |
|---|---|---|---|
| Vacuum | Up to 450°C | Preserved | Minimal sintering and surface area loss [92] |
| Static Air (Calcination) | 350-500°C | Significant Reduction | High variability and poor reproducibility [92] |
| Flowing Dry Air | 350°C | Moderate Reduction | Improved stability compared to static air [92] |
| Flowing Humid Air | 350°C | Accelerated Reduction | Presence of moisture accelerates sintering [92] |
Table 3: Comparison of Synthesis Methods for Alumina Nanoparticles
| Synthesis Method | Annealing Temperature | Phase Formed | Specific Surface Area (m²/g) |
|---|---|---|---|
| Co-precipitation | 750°C | γ-alumina | 206.2 [96] |
| Sol-Gel | 750°C | Largely Amorphous | 30.7 [96] |
Experimental Protocols for Optimized Thermal Treatment
Protocol: Stepwise Heat Treatment for Metal Oxide Nanocrystals
This protocol, adapted from a study on balancing ZnO crystallinity and SSA, uses a modified polymer-network gel precursor [93].
- Objective: To achieve high crystallinity while minimizing particle aggregation and surface area loss.
- Materials:
- Xerogel powder (e.g., ZnO xerogel from a polymer-network process)
- Tubular furnace with programmable temperature controller
- Thermogravimetric Analysis (TGA) instrument
- Procedure:
- Thermal Analysis: Perform TGA on the xerogel powder to identify key mass loss events and determine appropriate pre-calcination temperatures (e.g., 300°C for polymer burnout) [93].
- Pre-calcination: Place the xerogel powder in a crucible and load it into the furnace. Ramp the temperature to 300°C at a rate of 5°C/min and hold for 100 minutes. This step allows for the controlled removal of organics and release of thermal stress.
- High-Temperature Crystallization: Immediately after the pre-calcination hold, ramp the temperature to the final crystallization temperature (e.g., 650°C) at 5°C/min. Hold at this temperature for 200 minutes.
- Cooling: Allow the furnace to cool naturally to room temperature.
- Technical Notes: This stepwise method is designed to ensure a thorough release of thermal stress during annealing, which effectively reduces particle aggregation, leading to more single-crystal nanoparticles and a preserved surface area [93].
Protocol: Controlled Atmosphere Annealing for High Surface Area Anatase TiOâ‚‚
This protocol outlines methods to mitigate sintering during the annealing of high-surface-area TiOâ‚‚ [92].
- Objective: To preserve the surface area of anatase TiOâ‚‚ during thermal treatment.
- Materials:
- High-surface-area anatase TiOâ‚‚ powder
- Vacuum oven or tube furnace with gas flow capabilities
- Source of dry synthetic air
- Procedure - Vacuum Treatment:
- Load the TiOâ‚‚ powder into a vacuum oven.
- Evacuate the chamber and heat the sample to the target temperature (e.g., 450°C) under continuous vacuum.
- Hold for a specified duration (e.g., 2 hours).
- Procedure - Flow Condition Treatment:
- Load the TiOâ‚‚ powder into a tube furnace.
- Purge the tube with a continuous flow of dry synthetic air (e.g., 150 mL/min).
- Ramp the temperature to the target temperature (e.g., 350°C) and hold, maintaining the gas flow throughout the process.
- Technical Notes: Avoid static air calcination and the introduction of humidity, as both significantly accelerate sintering. Flow conditions improve reproducibility and stability compared to static air [92].
Workflow Diagram: Thermal Treatment Optimization Strategy
The following diagram outlines the logical decision-making process for selecting an appropriate thermal treatment protocol based on the target metal oxide and the application's primary requirement.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagent Solutions for Sol-Gel Synthesis and Thermal Treatment
| Item | Function / Application | Example from Literature |
|---|---|---|
| Tetraethyl Orthosilicate (TEOS) | Common silica precursor in sol-gel synthesis. | Used as the SiOâ‚‚ source in ZnO-SiOâ‚‚ nanocomposites [6]. |
| Zinc Acetate Dihydrate | Metal precursor for ZnO nanoparticle synthesis. | Preferred over nitrate-based precursors for yielding smoother surfaces [6]. |
| Titanium Alkoxides (e.g., Ti(iOPr)â‚„) | Standard precursors for sol-gel-derived TiOâ‚‚. | Forms the basis for TiOâ‚‚ photocatalyst studies [95] [9]. |
| Acetic Acid | Acid catalyst for controlled hydrolysis/polycondensation. | Used as a pH-controlling agent in SiOâ‚‚ synthesis to reduce defect density [6]. |
| Chelating Agents (e.g., Citric Acid, Tartaric Acid) | Promotes homogeneous metal distribution in polymer-gel methods. | Tartaric acid was used to chelate metal ions in a modified polymer-gel process for ZnO [93]. |
| Polymer Network Agents (Acrylamide/Bis-acrylamide) | Forms a 3D network to prevent particle aggregation during synthesis. | Created a homogeneous precursor gel for various metal oxide nanocrystals [93]. |
| Controlled Atmosphere Furnace | Enables thermal treatment under vacuum or specific gas flows. | Critical for preserving the surface area of anatase TiOâ‚‚ during annealing [92]. |
Optimizing the thermal treatment of sol-gel-derived materials is not a one-size-fits-all process but a strategic balance of time, temperature, and atmosphere. The protocols and data presented herein demonstrate that a stepwise heat treatment strategy and careful control of the thermal environment are highly effective in mitigating the crystallinity-surface area trade-off. By pre-calcining at an intermediate temperature to remove organics and relieve thermal stress, and by using flowing dry air or vacuum instead of static air, researchers can achieve metal oxide nanocrystals with both good crystallinity and preserved specific surface area.
Future directions in this field point toward more sophisticated and controlled processes. These include the use of hydrothermal treatments as an alternative to direct calcination for improved crystallization at lower temperatures [95] [97], and the development of advanced thermal protocols for creating complex multi-phase materials, such as glass-ceramics from 45S5 Bioglass, where controlled crystallization enhances chemical stability while maintaining bioactivity [94]. Continued refinement of these thermal strategies is essential for unlocking the full potential of sol-gel-synthesized metal oxides in advanced technological applications.
The sol-gel method is a cornerstone technique for synthesizing metal oxide photocatalysts, prized for its molecular-level control over composition, low processing temperatures, and ability to produce diverse nanostructures. [3] However, a significant challenge impeding the transition from laboratory research to practical application is the inherent instability and difficult recovery of powdered photocatalysts. These materials often suffer from particle aggregation, leading to a loss of active surface area, and are difficult to separate from treated water, causing secondary pollution and increasing operational costs. [98] [99]
To address these limitations, strategic material design is essential. This document details advanced methodologies centered on cross-linking strategies and the use of solid support materials to engineer robust, three-dimensional (3D) photocatalytic architectures. These approaches significantly enhance the mechanical integrity, chemical stability, and reusability of sol-gel-derived photocatalysts, thereby improving their viability for industrial-scale environmental remediation, including drug degradation in wastewater. [100] [99]
Cross-linking Strategies for Monolithic Gel Structures
Cross-linking transforms sol-gel-derived precursors into stable, 3D network structures that are inherently more manageable and reusable than powders. These strategies can be broadly classified into physical and chemical methods.
Physical Cross-linking
Physical cross-linking relies on non-covalent interactions, such as hydrogen bonding, van der Waals forces, and chain entanglements, to form reversible hydrogel networks.
- Mechanism and Materials: In the context of graphitic carbon nitride (g-C₃N₄) gels, nitrogen-rich functional groups (e.g., –NH₂, –NH–) on the polymer backbone form extensive hydrogen bonds with water molecules and other polymeric chains, creating a stable, water-swollen network. [99] Similarly, biomass precursors like chitosan and cellulose provide abundant hydroxyl and amino groups that facilitate physical gelation. [99]
- Advantages: This method is typically simple, avoids the use of harsh chemical cross-linkers, and results in gels that are often self-healing and injectable due to the reversible nature of the bonds.
Chemical Cross-linking
Chemical cross-linking involves the formation of permanent, covalent bonds between polymer chains, leading to more rigid and mechanically stable networks.
- Mechanism and Agents: This process creates an interconnected 3D network where the photocatalyst particles (e.g., g-C₃N₄, TiO₂, ZnO) are embedded within a stable polymeric matrix. Cross-linking agents directly bond with functional groups on the photocatalyst surface or the surrounding gel matrix. Common agents include:
- Epoxides: React with amino groups to form stable linkages.
- Genipin: A natural and less toxic alternative to synthetic cross-linkers.
- Metal Ions: Ions like Cu²⺠can coordinate with nitrogen atoms in g-C₃N₃, acting as ionic cross-linking centers. [99]
- Advantages: Chemically cross-linked gels exhibit superior mechanical strength, stability over a wider range of pH and temperature, and prolonged operational lifetime.
The following workflow outlines the general process for developing a cross-linked photocatalytic gel, from precursor selection to performance validation.
Gel Catalyst Development Workflow
Quantitative Performance of Cross-linked Gel Photocatalysts
The effectiveness of cross-linking strategies is demonstrated by enhanced photocatalytic performance and stability, as shown by studies on composite materials.
Table 1: Performance Metrics of Cross-linked and Composite Photocatalysts
| Photocatalyst Material | Target Pollutant | Degradation Efficiency | Reusability Cycles & Performance Retention | Key Enhancement Strategy |
|---|---|---|---|---|
| g-C₃N₄-based Hydrogel [99] | Methylene Blue | >90% in 60 min (visible light) | Not specified | 3D porous network for synergistic adsorption-degradation |
| Ag/TiOâ‚‚/CNT Composite [101] | Organic Dyes | >99% | 5 cycles with enhanced stability | Composite formation for charge separation |
| Fe–TiO₂–Bi₂O₃ [102] | Cephalexin | 96% in 120 min (UV) | Not specified | Heterojunction design & Fe³⺠doping |
| ZnFeâ‚‚Oâ‚„/ZnO/CeOâ‚‚ [103] | Erythrosine (ER) | 92.33% (visible light) | High potential for magnetic recovery | Ternary composite for synergistic effects & magnetic separation |
Support Materials and Composite Engineering
Incorporating sol-gel-derived photocatalysts onto or within inert support materials is a highly effective method to prevent aggregation, facilitate separation, and enhance mass transfer.
Silica (SiOâ‚‚) Matrices
Silica is an excellent support material due to its high surface area, thermal stability, and chemical inertness.
- Mechanism: A sol-gel derived SiO₂ matrix can encapsulate photocatalytic nanoparticles like ZnO, preventing their agglomeration and providing a high-surface-area environment. The formation of chemical bonds (e.g., Zn–O–Si in ZnO-SiO₂ composites) enhances structural integrity and can modify the electronic structure of the photocatalyst, improving charge separation. [6]
- Protocol: Synthesis of ZnO-SiOâ‚‚ Nanocomposites via Sol-Gel
- Step 1: SiO₂ Sol Preparation. Use a molar ratio of Tetraethyl Orthosilicate (TEOS) : Ethanol : H₂O = 1 : 10 : 4. Add acetic acid (0.05 mol per mol TEOS) as a catalyst. Stir vigorously at 300 rpm for 3 hours at 25°C. [6]
- Step 2: ZnO Incorporation. Pre-synthesize ZnO nanoparticles separately (e.g., from zinc acetate and NaOH in ethanol). [6] Disperse the ZnO nanoparticles into the SiOâ‚‚ sol under sonication to ensure homogeneity.
- Step 3: Gelation and Aging. Allow the mixture to gel at 75°C. Age the gel for 18 hours to strengthen the Si–O–Si network via Ostwald ripening. [6]
- Step 4: Drying and Annealing. Dry the gel at 120°C and subsequently anneal in the 700–900°C range to achieve the desired crystallinity and porosity. [6]
Magnetic Supports
Magnetic separation offers a facile and low-energy method for catalyst recovery.
- Mechanism: Integrating magnetic components like ZnFeâ‚‚Oâ‚„ (with a bandgap of ~1.9 eV) into a photocatalyst composite (e.g., ZnFeâ‚‚Oâ‚„/ZnO/CeOâ‚‚) allows the entire material to be recovered from an aqueous suspension using an external magnet. [103] This not only enables reusability but also prevents secondary pollution.
- Protocol: One-Pot Sol-Gel Auto-Combustion for ZnFeâ‚‚Oâ‚„/ZnO/CeOâ‚‚
- Step 1: Precursor Solution Preparation. Dissolve metal nitrate precursors of Zn, Fe, and Ce in stoichiometric ratios in deionized water. [103]
- Step 2: Fuel Addition. Use oxalic acid as a fuel agent. The fuel-to-oxidizer ratio is critical for controlling the exothermic reaction and the resulting particle size. [103]
- Step 3: Auto-Combustion. Heat the mixture on a hotplate until it ignites. The self-sustaining exothermic reaction produces a voluminous, fluffy powder. [103]
- Step 4: Calcination. Calcine the resulting powder to remove any residual organics and to crystallize the ternary oxide phases, forming the final nano-photocatalyst with a particle size of ~19 nm. [103]
Carbon-Based Supports and Hydrogels
Materials like graphitic carbon nitride (g-C₃N₄) and carbon nanotubes can be structured into gels or used as composite partners.
- Mechanism: Constructing 3D macroporous hydrogels or aerogels from g-C₃N₄ inhibits the aggregation of 2D nanosheets, dramatically increasing the exposure of active sites. The hierarchical porosity (macro- and mesopores) enhances mass transfer of pollutants to the active sites and facilitates light penetration. [100] [99]
- Advantages: These monolithic gels are easily retrievable from water, solving the recovery problem of powders. Their functional surfaces can be further modified with molecular imprinting techniques to enhance selectivity for specific pollutants like patulin. [100]
The following diagram illustrates how different support strategies contribute to the enhanced stability and reusability of the photocatalyst.
Support Material Mechanisms
The Scientist's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagent Solutions for Sol-Gel Catalyst Stabilization
| Reagent/Material | Function/Application | Example Use Case |
|---|---|---|
| Tetraethyl Orthosilicate (TEOS) | A common silica precursor for creating inert, high-surface-area support matrices. | Synthesis of ZnO-SiOâ‚‚ nanocomposites. [6] |
| Cyanamide / Dicyandiamide / Melamine | Nitrogen-rich precursors for the synthesis of graphitic carbon nitride (g-C₃N₄) structures. | Formation of g-C₃N₄-based hydrogels and aerogels. [99] |
| Chitosan / Cellulose | Natural biomass-derived polymers that serve as sustainable precursors and gelation agents. | Creating eco-friendly, nitrogen-doped gel frameworks. [99] |
| Oxalic Acid | A fuel agent used in sol-gel auto-combustion synthesis to control exothermic reactions. | Synthesis of ZnFeâ‚‚Oâ‚„/ZnO/CeOâ‚‚ ternary photocatalysts. [103] |
| Zinc Acetate Dihydrate | A common metal-organic precursor for the synthesis of ZnO nanostructures. | Preparation of ZnO nanoparticles and composites. [6] |
| Metal Nitrates (e.g., Fe, Zn, Ce) | Oxidizer precursors in sol-gel auto-combustion, providing metal cations for the final oxide. | One-pot synthesis of multi-metal oxide photocatalysts. [103] |
| Epoxides / Genipin | Chemical cross-linking agents to form covalent bonds within polymeric gel networks. | Enhancing the mechanical strength of g-C₃N₄-polymer hydrogels. [99] |
The strategic application of cross-linking and support materials represents a paradigm shift in the design of sol-gel-derived photocatalysts, moving from high-performance but unstable powders to robust, engineered materials suited for real-world applications. By creating 3D gel networks or composite structures with silica, magnetic components, or carbon-based materials, researchers can simultaneously address the critical challenges of nanoparticle aggregation, difficult recovery, and limited reusability. The protocols and data summarized herein provide a foundation for developing the next generation of stable, efficient, and recyclable photocatalytic systems for advanced environmental remediation.
Performance Evaluation and Comparative Analysis of Sol-Gel Derived Photocatalysts
Within the research on synthesizing metal oxide photocatalysts via the sol-gel method, comprehensive characterization is paramount for correlating synthesis parameters with the resulting material's structural, morphological, and optical properties. This document details standardized protocols for five pivotal characterization techniques: X-ray Diffraction (XRD), Scanning and Transmission Electron Microscopy (SEM/TEM), Fourier-Transform Infrared spectroscopy (FT-IR), Brunauer-Emmett-Teller analysis (BET), and UV-Visible Diffuse Reflectance Spectroscopy (UV-Vis DRS). These protocols are framed within the context of developing advanced photocatalytic materials, such as doped TiOâ‚‚, for applications like pharmaceutical pollutant degradation [104].
The following workflow outlines the logical sequence and primary objectives for characterizing a sol-gel-synthesized metal oxide photocatalyst.
Experimental Protocols & Application Notes
X-ray Diffraction (XRD)
Objective: To determine the crystallite phase, crystallinity, and average crystallite size of the synthesized metal oxide powder.
Detailed Protocol
- Sample Preparation: Finely grind the calcined powder using an agate mortar and pestle to minimize preferential orientation. Load the powder into a standard XRD sample holder and level the surface without applying excessive pressure to avoid texturing.
- Instrument Setup: Use a diffractometer with Cu Kα radiation (λ = 1.5406 Å). Set the operating voltage and current to 40 kV and 25 mA, respectively. A divergent and receiving slit of 1° is typical.
- Data Acquisition: Scan the sample over a 2θ range from 10° to 80° with a step size of 0.026° and a counting time of 0.5–1 second per step [105] [106].
- Data Analysis:
- Phase Identification: Compare the obtained diffraction pattern with standard reference patterns from the International Centre for Diffraction Data (ICDD) database.
- Crystallite Size Calculation: Apply the Debye-Scherrer equation to the full width at half maximum (FWHM) of the most intense peak:
- D = (K λ) / (β cosθ)
- Where D is the volume-weighted average crystallite size (nm), K is the shape factor (~0.9), λ is the X-ray wavelength, β is the FWHM of the diffraction peak (in radians), and θ is the Bragg angle [105].
Application Notes
- Sol-Gel Context: XRD is crucial for optimizing the calcination temperature of sol-gel precursors. For instance, a study on MgAl₂O₄ spinel showed weak, low-intensity peaks at 700°C, indicating incomplete crystallization, which sharpened and intensified after calcination at 900°C, confirming the formation of a pure, crystalline phase [105].
- Doping Analysis: XRD can detect structural changes due to doping. For Ce-doped TiOâ‚‚, a shift in diffraction peaks or a change in the unit cell volume can confirm the incorporation of Ce ions into the TiOâ‚‚ crystal lattice [104].
Table 1: Representative XRD Data from Sol-Gel Synthesized Materials
| Material | Calcination Temperature | Identified Crystal Phase | Average Crystallite Size (nm) | Reference |
|---|---|---|---|---|
| MgAl₂O₄ Spinel | 900 °C | Cubic Spinel | ~12 nm | [105] |
| {001}-TiO₂/Au | 450 °C | Anatase TiO₂ | Not Specified | [107] |
| Nb-doped ITO | 500 °C | Cubic Bixbyite | 5-10 nm | [107] |
| 60 SiO₂–34 CaO–4 MgO–2 P₂O₅ | 600-800 °C | Amorphous | - | [106] |
Scanning / Transmission Electron Microscopy (SEM/TEM)
Objective: To investigate the surface morphology, microstructure, particle size, and elemental composition of the photocatalyst.
Detailed Protocol
- Sample Preparation:
- For SEM: Dust a small amount of powder onto a conductive carbon tape adhered to an aluminum stub. Sputter-coat the sample with a thin (10–15 nm) layer of gold or carbon to prevent charging.
- For TEM: Disperse the powder in ethanol and sonicate for 10–15 minutes. Drop-cast a single drop of the suspension onto a lacey carbon-coated copper grid and allow it to dry in air.
- Instrument Setup:
- SEM: Use an accelerating voltage of 10–15 kV. Use a working distance of 8–10 mm. For elemental analysis, ensure the Energy Dispersive X-ray Spectroscopy (EDS) detector is calibrated.
- TEM: Use an accelerating voltage of 200 kV for high-resolution imaging.
- Data Acquisition:
- Acquire images at multiple magnifications to assess morphology and particle size distribution.
- Perform EDS analysis at multiple points or areas to determine elemental homogeneity and confirm doping.
- For TEM, acquire High-Resolution TEM (HR-TEM) images and Fast Fourier Transform (FFT) patterns to resolve lattice fringes and confirm crystallinity [107].
- Data Analysis: Use image analysis software (e.g., ImageJ) to measure particle sizes from multiple images. Analyze EDS spectra to identify elements present and their approximate atomic ratios.
Application Notes
- Morphology and Bioactivity: In hybrid SiOâ‚‚/PCL and TiOâ‚‚/PCL materials for bone implants, SEM confirmed a homogeneous structure, which is critical for bioactivity. After immersion in simulated body fluid, SEM/EDS was used to identify the formation of a calcium-phosphate (hydroxyapatite) layer on the material's surface, proving its bioactivity [108].
- Nanoparticle Distribution: TEM analysis of {001}-TiOâ‚‚/Au nanocomposites confirmed the successful distribution of Au colloids on the surface of the TiOâ‚‚ material, a key factor for its enhanced photocatalytic activity [107].
Fourier-Transform Infrared Spectroscopy (FT-IR)
Objective: To identify the surface functional groups, chemical bonds, and the formation of the inorganic network in sol-gel derived materials.
Detailed Protocol
- Sample Preparation: Use the KBr pellet method. Finely grind ~1–2 mg of the sample with 200–300 mg of anhydrous KBr. Press the mixture in a hydraulic press under vacuum at ~10 tons for 1–2 minutes to form a transparent pellet.
- Instrument Setup: Use an FT-IR spectrometer with a DTGS detector. Set the resolution to 4 cmâ»Â¹ and accumulate 45 scans per spectrum to ensure a good signal-to-noise ratio [108].
- Data Acquisition: Acquire the spectrum in the mid-infrared range, typically from 4000 to 400 cmâ»Â¹.
- Data Analysis: Identify characteristic absorption bands and correlate them with specific molecular vibrations. For example, in silica-based systems, the Si-O-Si stretching vibration appears at ~1080 cmâ»Â¹.
Application Notes
- Sol-Gel Process Monitoring: FT-IR is ideal for tracking the hydrolysis and condensation reactions during sol-gel synthesis. The disappearance of alkoxide (e.g., -OCH₃) bands and the appearance of broad -OH and metal-oxygen-metal (e.g., Ti-O-Ti, Si-O-Si) bands confirm the progression of the sol-gel reaction [109] [108].
- Hybrid Material Characterization: For organic-inorganic hybrids like PCL/SiO₂, FT-IR can reveal hydrogen bonding between the X–OH (X=Si, Ti) groups of the inorganic phase and the ester groups of the polymer, which is crucial for the formation of a homogeneous hybrid network [108].
Table 2: Key FT-IR Absorbance Bands in Sol-Gel Derived Materials
| Material / Bond | Wavenumber (cmâ»Â¹) | Vibration Mode | Significance |
|---|---|---|---|
| Si-O-Si | ~1080, ~800 | Stretching, Bending | Formation of silica network [108] |
| Ti-O-Ti | 400 - 800 | Stretching | Formation of titania network |
| O-H | ~3400 (broad) | Stretching | Surface hydroxyls / adsorbed water |
| C=O (PCL) | ~1720 | Stretching | Presence of polymer in hybrid [108] |
| P-O (Phosphate) | ~560, ~600 | Bending | Presence of phosphate groups [106] |
BET Surface Area Analysis (BET)
Objective: To determine the specific surface area, pore volume, and pore size distribution of the porous photocatalyst material.
Detailed Protocol
- Sample Preparation: Accurately weigh ~0.1–0.2 g of the calcined powder. Outgas the sample under vacuum at a temperature of 200 °C for 2–4 hours to remove any physically adsorbed contaminants and moisture [105] [106].
- Instrument Setup: Use an automated gas sorption analyzer with Nâ‚‚ as the adsorbate at its boiling point (77 K).
- Data Acquisition: Measure the Nâ‚‚ adsorption-desorption isotherm across a relative pressure (P/Pâ‚€) range from 0.01 to 0.99.
- Data Analysis:
- Surface Area: Apply the BET (Brunauer-Emmett-Teller) equation to the adsorption data in the relative pressure range of 0.05–0.30 to calculate the specific surface area (m²/g).
- Pore Characteristics: Use the BJH (Barrett-Joyner-Halenda) method on the desorption branch of the isotherm to determine the pore volume and pore size distribution.
Application Notes
- Thermal Treatment Impact: BET analysis is sensitive to calcination temperature. For a quaternary bioglass (60 SiO₂–34 CaO–4 MgO–2 P₂O₅), the surface area drastically decreased from 160.6 m²/g at 600 °C to 2.2 m²/g at 900 °C due to sintering and pore collapse, highlighting the importance of thermal control [106].
- Photocatalyst Performance: A high surface area, as seen in MgAlâ‚‚Oâ‚„ spinel with a mean pore size of 20.2 nm, provides numerous active sites for reactant adsorption, which is directly linked to enhanced performance in applications like hydrogen storage or photocatalytic degradation [105].
UV-Visible Diffuse Reflectance Spectroscopy (UV-Vis DRS)
Objective: To determine the optical absorption properties and band gap energy of the photocatalyst.
Detailed Protocol
- Sample Preparation: Place the powder sample in a holder equipped with a transparent window. Ensure a smooth, flat surface. A standard BaSOâ‚„ pellet is often used as a 100% reflectance reference.
- Instrument Setup: Use a UV-Vis spectrophotometer equipped with an integrating sphere. Scan the wavelength range from 250 to 800 nm [104].
- Data Acquisition: Collect the diffuse reflectance spectrum (R) of the sample relative to the reference.
- Data Analysis:
- Convert the reflectance data to the Kubelka-Munk function: F(R) = (1 - R)² / 2R.
- Plot [F(R)hν]^n versus hν (photon energy), where n is 1/2 for direct band gaps and 2 for indirect band gaps.
- Determine the band gap energy (Eg) by extrapolating the linear region of the plot to the x-axis ([F(R)hν]^n = 0).
Application Notes
- Doping Verification: DRS is the primary technique for confirming a redshift in the absorption edge of a doped photocatalyst. For Ce-doped TiOâ‚‚, increasing Ce content was shown to narrow the band gap, extending light absorption into the visible region (>400 nm), which is critical for harnessing solar energy [104].
- Material Screening: The band gap value allows researchers to quickly screen and select photocatalysts suitable for activation under specific light sources (UV or visible).
Table 3: Optical Band Gap Data from Sol-Gel Synthesized Photocatalysts
| Material | Analysis Technique | Band Gap Energy (eV) | Reference |
|---|---|---|---|
| Ce-doped TiOâ‚‚ | DRS | Lower than undoped TiOâ‚‚ | [104] |
| MgAlâ‚‚Oâ‚„ Spinel | DRS | 2.84 | [105] |
| Nb-doped ITO | Spectroscopic Ellipsometry | ~3.7 | [107] |
Research Reagent Solutions
Table 4: Essential Reagents for Sol-Gel Synthesis and Coating of Photocatalysts
| Reagent | Function / Application | Example Use Case |
|---|---|---|
| Tetraethyl Orthosilicate (TEOS) | Silicon (Si) network precursor for silica-based sols | Synthesis of MCM-41 mesoporous silica for drug delivery [107] |
| Titanium(IV) Isopropoxide (TIP) | Titanium (Ti) precursor for titania-based sols | Synthesis of Ce-TiOâ‚‚ photocatalytic films [104] |
| Acetylacetone (AcAc) | Chelating agent to control hydrolysis rate of metal alkoxides | Modification of TIP precursor in Ce-TiOâ‚‚ sol preparation [104] |
| Nitric Acid (HNO₃) | Acid catalyst to promote hydrolysis in the sol-gel process | Catalyst in the synthesis of quaternary bioglass [106] |
| Stearic Acid | Capping agent to prevent nanoparticle agglomeration | Synthesis of MgAlâ‚‚Oâ‚„ spinel nanoparticles [105] |
| Polycaprolactone (PCL) | Biocompatible polymer for forming organic-inorganic hybrids | Synthesis of SiOâ‚‚/PCL and TiOâ‚‚/PCL hybrid materials [108] |
The development of advanced photocatalysts via the sol-gel method requires robust quantification of performance through two fundamental metrics: quantum yield and degradation kinetics. Quantum yield represents the photon efficiency of a photocatalytic process, while degradation kinetics describe the rate at which pollutants are broken down. For researchers synthesizing metal oxide photocatalysts, accurate determination of these parameters is essential for comparing material performance, optimizing synthesis parameters, and advancing applications in environmental remediation and pharmaceutical degradation [110] [111].
The sol-gel synthesis method offers precise control over metal oxide composition, morphology, and structural properties, directly influencing both quantum efficiency and kinetic performance. This protocol details standardized methodologies for quantifying these critical performance metrics, with particular emphasis on sol-gel derived photocatalysts tested against pharmaceutical compounds and organic dyes commonly studied in water treatment research [112] [113] [18].
Theoretical Foundations
Quantum Yield (QY) Fundamentals
Quantum yield (Φ) quantifies the efficiency of photon utilization in photocatalytic processes, defined as the ratio of reaction events to photons absorbed. In semiconductor photocatalysis, this metric reflects how effectively a material converts incident light into photogenerated charge carriers that drive redox reactions [110].
The fundamental equation for apparent quantum yield (AQY) is:
AQY (%) = (Number of reaction events / Number of incident photons) × 100%
For photocatalytic degradation studies, this is typically expressed as: AQY = (Rate of pollutant degradation × Avogadro's number × Planck's constant × speed of light) / (Light intensity × irradiation area × wavelength × reaction time)
Recent research has demonstrated that AQY values can potentially exceed 100% under specific conditions due to multiple exciton generation or photo-thermal synergistic effects, challenging traditional assumptions about quantum efficiency limits [110].
Kinetic Model Foundations
Photocatalytic degradation typically follows pseudo-first-order kinetics with respect to pollutant concentration, as described by the Langmuir-Hinshelwood model:
-ln(C/Câ‚€) = kt
Where C is concentration at time t, Câ‚€ is initial concentration, and k is the apparent first-order rate constant. This model applies when the pollutant concentration is low compared to surface adsorption sites, and the degradation rate depends primarily on surface coverage [112] [114].
The initial degradation rate can be calculated as: râ‚€ = kCâ‚€
More complex kinetic models account for adsorption-desorption equilibrium, radical participation, and catalyst loading effects, providing deeper mechanistic insights [114].
Experimental Protocols for Efficiency Quantification
Photocatalytic Reactor Setup and Operation
Table 1: Standard Photocatalytic Reactor Configuration Components
| Component | Specification | Purpose |
|---|---|---|
| Light Source | UV (λ=365 nm) or visible (λ≥420 nm) LEDs/lamps with appropriate filters | Controlled photoexcitation of catalyst |
| Reaction Vessel | Quartz or Pyreactor with magnetic stirring | UV transparency and homogeneous mixing |
| Catalyst Concentration | 0.1-2.0 g/L (optimized for specific system) | Ensure sufficient active sites without light scattering |
| Pollutant Concentration | 5-20 mg/L (depending on compound molar absorptivity) | Representative concentration for kinetic modeling |
| Temperature Control | Water circulation jacket or temperature probe | Maintain isothermal conditions |
| Sampling Intervals | 0, 5, 10, 15, 30, 60, 90, 120 minutes | Adequate data points for kinetic modeling |
Procedure:
- Prepare pollutant solution at desired concentration in ultrapure water
- Add catalyst powder to solution and conduct adsorption-desorption equilibrium in dark for 30-60 minutes
- Take initial sample (t=0) before illumination
- Turn on light source and begin timing; record light intensity at reaction plane
- Withdraw samples at predetermined intervals and immediately centrifuge or filter to remove catalyst
- Analyze supernatant for pollutant concentration via HPLC or UV-Vis spectroscopy [112] [17] [18]
Quantum Yield Determination Protocol
Photon Flux Measurement:
- Calibrate light source using a silicon photodiode or chemical actinometer (ferrioxalate)
- Measure irradiance (W/m²) at multiple positions within reaction vessel
- Calculate photon flux (Einstein/L/s): I = Eλ/(hcNâ‚) Where E is irradiance (W/m²), λ is wavelength (m), h is Planck's constant, c is light speed, Nâ‚ is Avogadro's number
AQY Calculation:
- Perform degradation experiment under monochromatic light
- Determine initial degradation rate from linear portion of -ln(C/Câ‚€) vs. time plot
- Calculate AQY using: AQY = (r × N₠× h × c) / (I × A × λ × t) Where r is degradation rate (mol/s), I is light intensity (W/m²), A is irradiated area (m²), λ is wavelength (m), t is time (s) [110]
Critical Considerations:
- Use bandpass filters to ensure monochromatic illumination
- Measure light absorption by catalyst, not just incident light
- Account for reflection and scattering losses
- Maintain low pollutant conversion (<20%) for initial rate measurements
Data Analysis and Interpretation
Kinetic Parameter Extraction
Table 2: Exemplary Kinetic Data for Sol-Gel Synthesized Photocatalysts
| Photocatalyst | Pollutant | Rate Constant k (minâ»Â¹) | Degradation Efficiency (%) | Experimental Conditions |
|---|---|---|---|---|
| TOBP-3 (TiOâ‚‚/BiPOâ‚„) | Carbamazepine (10 mg/L) | 0.031 | ~98% (UV) | Catalyst: 0.5 g/L, pH: neutral [112] |
| ZnO (oxygen vacancy-rich) | Malachite Green | 0.028 | >90% (UV) | Catalyst: 1.0 g/L, pH: optimized [114] |
| 90TiO₂-10Fe₂O₃/PVP | Tetracycline HCl (10 ppm) | N/R | High activity (UV/Visible) | Catalyst: 0.1 g/L [18] |
| Cd₀.₅Zn₀.₅S | H₂ production | N/A | AQY=247% (optimized) | Temperature: 70°C, Low light intensity [110] |
Kinetic Analysis Steps:
- Plot -ln(C/Câ‚€) versus irradiation time
- Perform linear regression on the initial linear portion (typically up to 70-80% degradation)
- Calculate regression coefficient (R²) to confirm fit quality
- Determine rate constant k from slope of the linear fit
- Calculate half-life: tâ‚/â‚‚ = ln(2)/k [112] [114]
Advanced Kinetic Modeling
For more complex systems, additional kinetic parameters can be extracted:
Langmuir-Hinshelwood Model: r = káµ£KC/(1 + KC) Where káµ£ is intrinsic rate constant, K is adsorption constant
Determination of Radical Contributions:
- Conduct scavenger studies to identify primary reactive species
- Use specific quenchers: isopropanol (•OH), EDTA (hâº), p-benzoquinone (•Oâ‚‚â»)
- Calculate contribution percentages from rate suppression [112] [103]
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for Photocatalytic Efficiency Studies
| Reagent/Solution | Composition/Preparation | Primary Function | Application Notes |
|---|---|---|---|
| Pollutant Stock Solution | Dissolve reference compound (carbamazepine, methylene blue, tetracycline) in DI water | Standardized substrate for degradation studies | Typical concentration: 100-1000 mg/L; store at 4°C protected from light [112] [17] [18] |
| Radical Scavengers | 1M solutions in DI water: isopropanol (•OH), EDTA (hâº), p-benzoquinone (•Oâ‚‚â»), sodium azide (¹Oâ‚‚) | Mechanistic studies to identify dominant reactive species | Add small volumes (10-100 μL) to reaction mixture; use individually and in combination [112] [103] |
| pH Buffer Solutions | Phosphate (pH 5-8), acetate (pH 3-6), borate (pH 8-10) at 0.1M concentration | Control solution pH for adsorption and reactivity studies | Verify buffer components do not participate in reactions or absorb significantly at working wavelengths [114] |
| Chemical Actinometer | Potassium ferrioxalate (0.15M) for UV, Reinecke's salt for visible range | Photon flux quantification for quantum yield calculation | Prepare fresh; calibrate against standard reference [110] |
| Catalyst Dispersion Aid | 0.1% surfactant solutions (Triton X-100, SDS) or ultrasonication protocol | Improve catalyst dispersion and maintain suspension | Use minimal surfactant to avoid interference with degradation reactions [17] |
Workflow Visualization
Photocatalytic Efficiency Assessment Workflow
Troubleshooting and Optimization Guidelines
Low Quantum Yield:
- Verify light source calibration and photon flux calculations
- Ensure monochromatic light using appropriate filters
- Check catalyst dispersion and light penetration depth
- Confirm pollutant concentration is within linear range for kinetics
Poor Kinetic Fitting:
- Extend adsorption-equilibrium period in dark
- Verify sampling represents true reaction quench
- Check for intermediate products that may absorb at analysis wavelength
- Consider mass transfer limitations at high catalyst loading
Enhancement Strategies:
- Incorporate oxygen vacancies to improve charge separation [114]
- Design heterojunctions to reduce electron-hole recombination [112] [103]
- Optimize catalyst calcination temperature to balance crystallinity and surface area [17] [115]
- Tune bandgap through composite formation for visible light activation [18] [103]
Standardized quantification of quantum yield and degradation kinetics enables meaningful comparison of photocatalytic materials across research studies. For sol-gel synthesized metal oxides, these metrics directly correlate with synthesis parameters and structural properties, guiding rational catalyst design. The protocols outlined provide a framework for rigorous efficiency assessment, particularly relevant for pharmaceutical degradation and water treatment applications where reproducible performance data is essential for technology advancement.
The pursuit of efficient photocatalytic materials for environmental remediation and renewable energy is a cornerstone of modern materials science. Titanium dioxide (TiOâ‚‚) and zinc oxide (ZnO) are among the most widely studied metal oxide semiconductors for these applications, prized for their stability, non-toxicity, and photocatalytic activity. However, their large bandgaps and tendency for rapid charge carrier recombination limit their practical efficiency [116] [117]. Composite systems, particularly those synthesized via the versatile sol-gel method, have emerged as a powerful strategy to overcome these limitations by enhancing light absorption, improving charge separation, and increasing stability [6] [118]. This Application Note provides a comparative analysis of the performance of TiOâ‚‚, ZnO, and their composite systems, framed within doctoral research on sol-gel synthesis of metal oxide photocatalysts. It includes structured quantitative data, detailed experimental protocols, and essential resource guidance for researchers and scientists.
Performance Comparison and Key Findings
The photocatalytic performance of TiOâ‚‚, ZnO, and their composites is highly dependent on their intrinsic properties and the conditions under which they are tested. The following table summarizes key performance metrics and characteristics as reported in recent literature.
Table 1: Comparative performance of TiOâ‚‚, ZnO, and selected composite photocatalysts.
| Photocatalyst | Synthesis Method | Target Pollutant/Application | Performance Metric | Key Findings/Enhancement Mechanism | Ref. |
|---|---|---|---|---|---|
| TiOâ‚‚ Nanoparticles | Sol-Gel | Methylene Blue (MB) | ~96.6% degradation in 240 min under UV | Flake-like structures (avg. 52 nm); band gap ~3.19 eV; mixed anatase/rutile phase. | [119] |
| TiO₂–SiO₂ Composite | Sol-Gel | Rhodamine B (RhB) & Methylene Blue (MB) | ~92% (RhB, 6h) & ~70% (MB, 5h) degradation under light. | Point of zero charge (pHpzc) = 6.4; concentration-dependent biological activity. | [33] |
| ZnO–SiO₂ (10:90) | Sol-Gel | Methylene Blue (MB) | Highest degradation rate among composites with varying ZnO loadings. | Optimal structural defects (oxygen vacancies) narrow band gap; inhibits ZnO aggregation. | [118] |
| ZnO–SiO₂ Composite | Sol-Gel | Structural Analysis | N/A | Formation of Zn–O–Si bonds confirmed by FT-IR; homogeneous elemental distribution. | [6] |
| TiOâ‚‚/CuO Composite | Sol-Gel (Comparative study) | Herbicide Imazapyr | Highest photonic efficiency among TiOâ‚‚-based composites. | Enhanced light absorption and superior charge separation. | [116] |
| NiTiO₃/TiOâ‚‚ Composite | Sol-Gel | Hydrogen Production | Hâ‚‚ evolution rate of 9.74 μmol minâ»Â¹ (17.1% improvement over pristine NiTiO₃). | Type-II heterojunction reduces charge recombination by 85%. | [120] |
Analysis of Comparative Data
The data reveals that while pure oxides like TiO₂ can achieve high degradation efficiencies [119], composite systems consistently demonstrate enhanced or more stable performance. The superiority of composites like TiO₂/CuO and ZnO–SiO₂ is attributed to the formation of heterojunctions, which facilitate the separation of photogenerated electrons and holes, thereby increasing the quantum efficiency of the photocatalytic process [116] [118]. Furthermore, the incorporation of a support matrix like SiO₂ inhibits the agglomeration of active nanoparticles, provides a high surface area for pollutant adsorption, and can enhance chemical stability [6] [118].
Detailed Experimental Protocols
The sol-gel method is a cornerstone technique for synthesizing these materials, offering fine control over composition, morphology, and crystallinity at relatively low cost [119] [6]. Below are detailed protocols for key experiments cited in this analysis.
Protocol: Synthesis of TiO₂–SiO₂ Mixed Oxide via Sol-Gel
This protocol is adapted from the synthesis used to produce a composite with dual-functional catalytic and biological properties [33].
- Objective: To synthesize a TiO₂–SiO₂ mixed oxide nanocomposite for photocatalytic degradation of dyes (Rhodamine B and Methylene Blue).
- Materials:
- Precursors: Titanium isopropoxide (TTIP, 0.0101 mol) and Tetraethyl orthosilicate (TEOS, 0.0224 mol).
- Solvents/Catalysts: Ethanol, deionized water, and a catalyst (e.g., HCl or acetic acid).
- Procedure:
- Sol Preparation: Synthesize TiOâ‚‚ and SiOâ‚‚ sols separately. For the SiOâ‚‚ sol, hydrolyze TEOS in ethanol with water under acidic catalysis.
- Mixing: Combine the TiOâ‚‚ and SiOâ‚‚ sols in a 1:1 ratio and stir vigorously to ensure homogeneity.
- Gelation & Aging: Allow the mixed sol to gel at room temperature, then age the gel for a specific duration (e.g., 24 hours) to strengthen the network.
- Drying & Calcination: Dry the gel at an elevated temperature (e.g., 100-120°C) to remove solvents, followed by calcination (e.g., 450-600°C) to crystallize the TiO₂ phase.
- Characterization: The synthesized nanomaterial was characterized using UV-Vis spectroscopy, SEM, EDX, FT-IR, DSC, and TGA [33].
Protocol: Synthesis of Defect-Engineered ZnO/SiOâ‚‚ Composites
This protocol focuses on creating composites where structural defects in ZnO enhance photocatalytic activity [118].
- Objective: To synthesize ZnO/SiOâ‚‚ composites with varying ZnO loadings and investigate the effect of structural defects on MB degradation.
- Materials:
- Precursors: Zinc acetate dihydrate and Tetraethyl orthosilicate (TEOS).
- Other Chemicals: Ethanol, distilled water, hydrochloric acid (HCl, 37%) as a catalyst.
- Procedure:
- SiOâ‚‚ Sol Formation: Hydrolyze TEOS in a mixture of ethanol and distilled water, using HCl to adjust the pH to ~2. Stir for 1 hour.
- ZnO Incorporation: Add zinc acetate dihydrate to the SiOâ‚‚ sol in varying mass ratios (e.g., 5, 10, 15% ZnO). Stir the mixture for 3 hours to form a homogeneous sol.
- Gelation & Aging: Let the sol stand until it transforms into a gel. Age the gel for 24 hours.
- Drying & Calcination: Dry the gel at 100°C for 12 hours and subsequently calcine it at 600°C for 3 hours.
- Characterization: Use XRD to determine crystallite size and dislocation density. Employ UV-Vis DRS to calculate the band gap and FT-IR to confirm the formation of Zn–O–Si bonds [118].
Workflow Diagram: Sol-Gel Synthesis and Photocatalytic Testing
The following diagram illustrates the general workflow for the synthesis, characterization, and performance evaluation of sol-gel derived photocatalysts, integrating the key steps from the protocols above.
Charge Transfer Mechanisms in Composite Systems
The enhanced performance of composite systems is fundamentally rooted in efficient charge separation at the heterojunction interfaces. The diagram below illustrates the type-II heterojunction mechanism, which is a common and effective charge separation pathway in many composite photocatalysts.
This mechanism, as reported for systems like NiTiO₃/TiO₂, involves the spatial separation of photogenerated electrons and holes across the interface of two semiconductors with staggered band structures, drastically reducing recombination and enhancing photocatalytic activity [120].
The Scientist's Toolkit: Essential Research Reagents
The following table lists key reagents and materials commonly used in the sol-gel synthesis of TiOâ‚‚, ZnO, and their composites, as referenced in the provided studies.
Table 2: Essential reagents for sol-gel synthesis of metal oxide photocatalysts.
| Reagent/Material | Typical Function | Example Use Case |
|---|---|---|
| Titanium Isopropoxide (TTIP) | Titanium precursor for TiO₂ sol-gel synthesis. | Primary TiO₂ source in TiO₂–SiO₂ composite synthesis [33]. |
| Zinc Acetate Dihydrate | Zinc precursor for ZnO nanoparticle formation. | Used in the synthesis of ZnO and ZnO–SiO₂ nanocomposites [6] [118]. |
| Tetraethyl Orthosilicate (TEOS) | Silicon precursor for forming the SiO₂ matrix. | Source of SiO₂ in TiO₂–SiO₂ and ZnO–SiO₂ composites [33] [6] [118]. |
| Ethanol / 2-Propanol | Solvent for homogenizing precursors and controlling reaction kinetics. | Common solvent in sol-gel processes; used in almost all cited protocols. |
| Hydrochloric Acid (HCl) / Acetic Acid | Catalyst for controlling hydrolysis and condensation rates. | Acid catalyst for TEOS hydrolysis in SiOâ‚‚ and composite synthesis [6] [118]. |
| Sodium Hydroxide (NaOH) | Precipitating agent for base-catalyzed synthesis routes. | Used for precipitating ZnO nanoparticles from zinc salt solutions [6]. |
Within the broader scope of thesis research on the sol-gel method for metal oxide photocatalyst synthesis, this document details application notes and protocols for evaluating photocatalytic dye degradation. The sol-gel technique is a cornerstone of this research due to its exceptional capability to produce metal oxide nanostructures with tailored compositions, homogeneous dopant distribution, and controlled morphologies at relatively low processing temperatures [3] [6]. These characteristics are paramount for engineering photocatalysts with optimized surface reactivity and charge carrier dynamics for environmental remediation [7] [121].
This protocol focuses on degrading two model dye pollutants: Methylene Blue (MB) and Rhodamine B (RhB). These dyes are significant contaminants in industrial wastewater, and their degradation serves as a standard benchmark for photocatalytic activity [122]. The procedures herein are designed to be conducted using sol-gel-synthesized metal oxide photocatalysts, with specific examples including ZnO-based systems and heterojunction composites like Znâ‚‚SnOâ‚„/SnOâ‚‚ [122] [123].
Experimental Protocols
Synthesis of Metal Oxide Photocatalysts via Sol-Gel Method
The sol-gel method enables precise molecular-level control over the photocatalyst's properties. The following protocol, inspired by the synthesis of ZnO-SiOâ‚‚ nanocomposites and other metal oxides, can be adapted for various single and composite metal oxides [3] [6].
Title: Sol-Gel Synthesis Workflow
- Objective: To synthesize ZnO nanoparticles with controlled crystallinity and surface area.
- Materials:
- Zinc acetate dihydrate (Zn(CH₃COO)₂·2H₂O)
- Ethanol (Câ‚‚Hâ‚…OH)
- Sodium hydroxide (NaOH) pellets
- Deionized water
- Procedure:
- Precursor Solution: Dissolve 10 g of zinc acetate dihydrate in 100 mL of ethanol under vigorous stirring (e.g., 300 rpm) at room temperature.
- Precipitation: Gradually add 1 M NaOH solution to the precursor solution to initiate precipitation. The use of ethanol, instead of water, slows the reaction, promoting controlled growth and potentially favoring nanorod morphologies [6].
- Aging: Allow the mixture to age for 24 hours to ensure the complete formation of zinc hydroxide intermediates.
- Thermal Decomposition: Anneal the resulting precipitate at 450 °C in a muffle furnace. This step decomposes the precursor into crystalline ZnO and removes residual organics, typically yielding crystallites smaller than 50 nm [6].
- Objective: To create a composite material where the SiOâ‚‚ matrix inhibits ZnO aggregation, enhances charge separation, and improves chemical stability.
- Materials:
- Tetraethyl orthosilicate (TEOS)
- Ethanol (Câ‚‚Hâ‚…OH)
- Acetic acid (CH₃COOH)
- Pre-synthesized ZnO nanoparticles (as above) or zinc salt.
- Procedure:
- SiOâ‚‚ Sol Preparation: Prepare a mixture with a molar ratio of TEOS:EtOH:Hâ‚‚O of 1:10:4. Add acetic acid (0.05 mol per mol of TEOS) as a catalyst to control the hydrolysis and polycondensation rates [6].
- Stirring and Gelation: Stir the mixture vigorously for 3 hours at 25°C. Then, heat it to 75 °C to initiate gelation, which helps achieve a uniform pore distribution.
- Aging: Age the gel for 18 hours to strengthen the Si–O–Si network.
- Composite Formation: Incorporate the ZnO nanoparticles into the SiOâ‚‚ sol before gelation, or co-precipitate using suitable zinc and silicon precursors.
- Annealing: Anneal the composite gel at temperatures ranging from 700–900 °C. Higher temperatures promote crystallinity of the ZnO phase while the SiO₂ matrix may remain largely amorphous [6]. FT-IR analysis confirms successful composite formation through the presence of Zn–O–Si bonds [6].
Photocatalytic Dye Degradation Assay
This standard protocol evaluates the performance of synthesized photocatalysts in degrading MB and RhB dyes.
Title: Dye Degradation Experimental Workflow
- Objective: To quantify the degradation efficiency of a photocatalyst against MB and RhB dyes under illumination.
- Materials:
- Photocatalyst powder (e.g., sol-gel synthesized ZnO, ZnO-SiOâ‚‚, or other composites)
- Methylene Blue (MB) dye
- Rhodamine B (RhB) dye
- Deionized water
- Photoreactor (or beaker) with magnetic stirrer
- Light source (Xe lamp simulating solar spectrum, UV lamp, or natural sunlight)
- UV-Vis Spectrophotometer
- Procedure:
- Solution Preparation: Prepare an aqueous dye solution with a known concentration (e.g., 10 mg/L for MB or RhB).
- Adsorption-Desorption Equilibrium: To a volume of the dye solution, add a specific amount of photocatalyst (e.g., 50 mg [123]). Place the mixture in the photoreactor and stir in the dark for 30-60 minutes to establish adsorption-desorption equilibrium between the dye and the catalyst surface [122].
- Illumination: Turn on the light source to initiate the photocatalytic reaction. Maintain constant stirring throughout the experiment.
- Sampling: At regular time intervals, withdraw a small aliquot of the suspension.
- Analysis: Centrifuge the sample to remove catalyst particles. Measure the absorbance of the clear supernatant using a UV-Vis spectrophotometer. The maximum absorbance for MB is around 664 nm, and for RhB, it is approximately 554 nm.
- Calculation: The photocatalytic degradation efficiency (η) at time t is calculated as: η (%) = [(C₀ - Cₜ) / C₀] × 100% where C₀ is the initial concentration after dark adsorption, and Cₜ is the concentration at time t.
Performance Data and Analysis
The performance of photocatalysts is evaluated based on their degradation efficiency under specific conditions. The tables below summarize key performance metrics from recent studies.
Table 1: Photocatalytic performance of various catalysts against single-dye systems.
| Photocatalyst | Synthesis Method | Target Dye | Catalyst Dose | Light Source | Time (min) | Degradation Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| Znâ‚‚SnOâ‚„/SnOâ‚‚ | Solid-state | Methylene Blue (MB) | 50 mg | Natural Sunlight | 120 | 99.1% | [123] |
| Znâ‚‚SnOâ‚„/SnOâ‚‚ | Solid-state | Rhodamine B (RhB) | 50 mg | Natural Sunlight | 120 | 70.6% | [123] |
| WO₃/BaTiO₃ (W/BT5) | Not Specified | Rhodamine B (RhB) | Not Specified | Visible Light | Not Specified | 1.25x vs. WO₃* | [124] |
| WO₃/BaTiO₃ (W/BT5) | Not Specified | Methylene Blue (MB) | Not Specified | Visible Light | Not Specified | 1.38x vs. WO₃* | [124] |
Table 2: Performance in multicomponent dye systems and key influencing factors.
| System / Factor | Photocatalyst | Experimental Conditions | Key Result / Observation | Reference |
|---|---|---|---|---|
| Multicomponent (MB + RhB) | Znâ‚‚SnOâ‚„/SnOâ‚‚ | 15 mg catalyst, Natural Sunlight, 120 min | MB: 97.9%; RhB: 53.2% (shows competitive degradation) | [123] |
| Critical Performance Factors | Various Metal Oxides | --- | Key factors: surface area, crystallinity, defect engineering, heterojunction formation, pH, temperature | [7] [121] |
| Role of Heterojunctions | WO₃/BaTiO₃ | --- | Improved charge carrier separation reduces electron-hole recombination, boosting efficiency. | [124] |
*Performance reported as enhancement factor relative to pure WO₃.
The Scientist's Toolkit: Reagents and Materials
Table 3: Essential research reagents and materials for sol-gel synthesis and dye degradation studies.
| Item | Function / Application | Brief Explanation |
|---|---|---|
| Zinc Acetate Dihydrate | Precursor for ZnO synthesis | A common metal salt precursor that provides Zn²⺠ions for the formation of ZnO nanoparticles during sol-gel processing [6]. |
| Tetraethyl Orthosilicate (TEOS) | Precursor for SiOâ‚‚ matrix | The most common silicon alkoxide used in sol-gel chemistry to form a silica (SiOâ‚‚) network, which can act as a support matrix in composites [6]. |
| Sodium Hydroxide (NaOH) | Precipitating and pH-controlling agent | Used to basify the solution, facilitating the hydrolysis and polycondensation of precursors and the precipitation of metal hydroxides/oxides [6]. |
| Methylene Blue (MB) | Model cationic dye pollutant | A thiazine dye used as a standard benchmark to evaluate and compare the photocatalytic oxidation performance of synthesized catalysts [122]. |
| Rhodamine B (RhB) | Model cationic dye pollutant | A xanthene dye used as a standard benchmark for photocatalytic activity tests under visible light [122]. |
| Acetic Acid | Catalyzing agent for SiOâ‚‚ sol | Used as a catalyst in the sol-gel synthesis of SiOâ‚‚ to control the reaction rate and reduce defect density, leading to better structural integrity [6]. |
Mechanisms and Pathways
Photocatalytic Mechanism
The general mechanism of dye degradation on a metal oxide photocatalyst surface involves multiple steps that generate highly reactive radical species.
Title: Photocatalytic Dye Degradation Mechanism
- Photoexcitation: Upon absorbing light with energy equal to or greater than its bandgap, the photocatalyst (e.g., ZnO) generates electron-hole pairs (e⻠in the conduction band and h⺠in the valence band) [122] [125].
- Charge Migration and Recombination: The photogenerated carriers migrate to the surface. A key challenge is their recombination, which releases energy as heat or light and reduces efficiency. Strategies like forming heterojunctions (e.g., Znâ‚‚SnOâ‚„/SnOâ‚‚) are employed to spatially separate electrons and holes, thereby suppressing recombination [123] [125].
- Surface Redox Reactions: The holes and electrons drive redox reactions with adsorbed species. Holes can directly oxidize dye molecules or react with water to generate hydroxyl radicals (•OH). Electrons reduce dissolved oxygen to form superoxide radical anions (O₂•â»), which subsequently produce other reactive oxygen species [122] [125].
- Dye Mineralization: These highly reactive radicals (primarily •OH) non-selectively attack and break down the complex organic structure of the dyes (MB, RhB) into simpler molecules, ultimately leading to complete mineralization into CO₂, H₂O, and inorganic ions [122] [123].
Dye Degradation Pathways
- Methylene Blue: Degradation primarily occurs through N-demethylation, where methyl groups are sequentially removed from the amino groups, forming Azure A, B, and C as intermediates, before cleavage of the aromatic ring structure [123].
- Rhodamine B: Degradation typically proceeds through a sequential de-ethylation process, where each ethyl group on the amino moiety is removed, forming a series of intermediates. This is followed by the destruction of the conjugated xanthene ring, leading to complete decolorization and mineralization [123].
Metal oxide nanomaterials synthesized via the sol-gel method represent a promising class of multifunctional agents for biomedical and environmental remediation applications. Their unique physicochemical properties—including high surface area, tunable bandgap, and surface reactivity—enable efficient photocatalytic degradation of pharmaceutical pollutants and confer inherent antibacterial activity. This application note provides a structured assessment of these functionalities, detailing quantitative performance data and standardized experimental protocols suitable for researchers and drug development professionals. The content is contextualized within broader thesis research on sol-gel synthesized metal oxide photocatalysts, focusing on reproducible methodologies for evaluating key biological and catalytic properties.
Experimental Protocols & Workflows
Sol-Gel Synthesis of Metal Oxide Photocatalysts
The sol-gel process enables precise control over stoichiometry, morphology, and crystallinity at low processing temperatures. The following generalized protocol, synthesizable for various metal oxide systems, is adapted from specific examples for TiO2–SiO2 and Fe-doped SrTiO3 [33] [126].
Protocol: Synthesis via Organic Sol-Gel Route
- Reagents: Titanium(IV) isopropoxide (TTiP, ≥97.0%), Tetraethyl orthosilicate (TEOS, for SiO2), Strontium nitrate (Sr(NO3)2·4H2O, p.a.), Iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, p.a.), absolute ethanol, citric acid monohydrate, ammonium solution (NH3).
- Equipment: Three-neck round-bottom flask, magnetic stirrer with heating, reflux condenser, drying oven, muffle furnace.
- Procedure:
- Solution Preparation: Prepare three separate solutions.
- Solution A: For TiO2-SiO2, mix TTIP (0.0101 mol) and TEOS (0.0224 mol) in ethanol. For SrTiO3, dissolve Ti(OBu)4 in ethanol with citric acid under vigorous stirring [33] [126].
- Solution B: Dissolve the cation precursor (e.g., Sr(NO3)2 or Fe(NO3)3) in ethanol at a 1:1 molar ratio.
- Solution C: A chelating agent like citric acid may be used in ethanol.
- Mixing & Gelation: Slowly add Solution B to Solution A under vigorous stirring. Adjust pH if necessary using NH3. The mixture will gradually transform into a translucent gel.
- Aging: Allow the gel to age in air for 24–48 hours to complete hydrolysis and polycondensation, forming an M–O–M network [127].
- Drying: Dry the aged gel at 80–120°C for 12–24 hours to remove the solvent.
- Calcination: Heat the dried gel in a muffle furnace at a predetermined temperature (e.g., 500–700°C for 2–4 hours) to crystallize the metal oxide phase and remove organic residues. The heating rate should be controlled (e.g., 5°C/min) [128].
- Solution Preparation: Prepare three separate solutions.
The workflow for material synthesis and subsequent bio-catalytic assessment is summarized in the diagram below.
Diagram 1: Experimental workflow for synthesis and assessment.
Protocol for Antibacterial Activity Assessment
The disk diffusion or broth dilution methods can evaluate the antibacterial efficacy of synthesized powders against relevant strains, such as E. coli and S. aureus [126] [127].
Protocol: Broth Dilution Method for MIC Determination
- Reagents: Mueller-Hinton Broth (MHB), sterile saline solution (0.85% NaCl), test bacterial strains (e.g., E. coli ATCC 25922, S. aureus ATCC 25923).
- Equipment: Sterile 96-well microtiter plates, incubator, spectrophotometer.
- Procedure:
- Bacterial Inoculum: Adjust the turbidity of a fresh bacterial culture in saline to a 0.5 McFarland standard (~1.5 × 10^8 CFU/mL). Dilute in MHB to achieve a working concentration of ~5 × 10^5 CFU/mL.
- Nanomaterial Dispersion: Prepare a stock dispersion of the photocatalyst powder in sterile water and sonicate for 30 minutes. Serially dilute the stock in MHB across the microtiter plate wells.
- Inoculation & Incubation: Add an equal volume of the bacterial inoculum to each well, resulting in a final test volume of 200 µL per well. Include growth control (bacteria + MHB) and sterility control (MHB only) wells.
- Incubation: Seal the plate and incubate at 37°C for 18–24 hours.
- Analysis: Determine the Minimum Inhibitory Concentration (MIC) visually as the lowest concentration that inhibits visible growth. Alternatively, measure optical density at 600 nm (OD₆₀₀) for quantitative assessment.
The proposed mechanism of antibacterial action is visualized below.
Diagram 2: Proposed antibacterial mechanism of action.
Protocol for Photocatalytic Drug Degradation
This protocol assesses the catalyst's efficiency in degrading antibiotic pollutants under light irradiation [126] [128].
Protocol: Batch Photocatalytic Degradation of Antibiotics
- Reagents: Target antibiotic (e.g., Tetracycline, Amoxicillin), photocatalyst powder, deionized water, 0.1 M HCl and 0.1 M NaOH for pH adjustment.
- Equipment: Batch photoreactor (e.g., Pyrex glass cell), magnetic stirrer, light source (e.g., 6 W UVC lamp or simulated solar light), UV-Vis spectrophotometer or HPLC system.
- Procedure:
- Reaction Mixture: Add a specific catalyst load (e.g., 0.1–1.0 g/L) to an aqueous solution of the antibiotic (e.g., 10–100 mg/L) in the photoreactor.
- Adsorption-Desorption Equilibrium: Stir the mixture in the dark for 30–60 minutes to establish adsorption-desorption equilibrium.
- Irradiation: Turn on the light source to initiate the photocatalytic reaction. Maintain constant stirring and temperature (e.g., with a water circulation jacket).
- Sampling: At regular time intervals, withdraw aliquots of the suspension and centrifuge or filter (0.22 µm membrane) to remove catalyst particles.
- Analysis: Quantify the remaining antibiotic concentration in the filtrate using a calibrated UV-Vis spectrophotometer (e.g., at λmax = 357 nm for Tetracycline) or HPLC. Calculate degradation efficiency as ( \frac{C0 - Ct}{C0} \times 100\% ), where ( C0 ) and ( C_t ) are concentrations at time zero and t, respectively.
The photocatalytic degradation process is based on the generation of reactive oxygen species.
Diagram 3: Photocatalytic degradation mechanism.
Quantitative Performance Data
The following tables summarize key performance metrics from recent studies for direct comparison.
Table 1: Antibacterial Activity of Sol-Gel Derived Metal Oxides
| Photocatalyst Material | Bacterial Strain(s) | Key Experimental Conditions | Performance Outcome | Reference |
|---|---|---|---|---|
| Fe-doped SrTiO3 (SrTi({0.15})Fe({0.85})O(_3)) | E. coli ATCC 25922, P. aeruginosa ATCC 27853 | Not specified (powder tested) | Antibacterial efficiency greater than undoped SrTiO3 | [126] |
| Nb2O5-containing nanosized powders (TiO2/TeO2/Nb2O5) | E. coli ATCC 25922, P. aeruginosa ATCC 27853, S. aureus ATCC 25923 | Concentration-dependent testing; combined with antibiotic ciprofloxacin for E. coli | Exhibited antibacterial activity against all tested bacterial strains; showed synergistic, concentration-dependent enhancement with ciprofloxacin against E. coli. | [127] |
| TiO2–SiO2 nanocomposite | Tested using human red blood cells (HRBCs) for cytotoxicity | Clear concentration-dependent trend | All assessed biological activities increased with higher nanocomposite concentrations. | [33] |
Table 2: Photocatalytic Degradation of Pharmaceuticals
| Photocatalyst Material | Target Pollutant | Key Experimental Conditions | Degradation Efficiency & Performance | Reference |
|---|---|---|---|---|
| TiO2–SiO2 Nanocomposite | Rhodamine B (RhB) dye | Light irradiation, 6 hours | ~92% degradation | [33] |
| TiO2–SiO2 Nanocomposite | Methylene Blue (MB) dye | Light irradiation, 5 hours | ~70% degradation | [33] |
| Fe-doped SrTiO3 (Undoped - STO) | Tetracycline Hydrochloride (TCH) | UV and Visible light irradiation | Better photocatalytic activity compared to Fe-doped sample under both light sources | [126] |
| Fe-doped SrTiO3 (SrTi({0.15})Fe({0.85})O(_3)) | Malachite Green (MG) dye | Visible light irradiation | Superior photocatalytic activity under visible light | [126] |
| TiO2/WO3 | Amoxicillin | Solar pilot-scale reactor, 0.1 g/L catalyst load, 100 ppm AMX | 64.4% amoxicillin removal; treated solution exhibited lower antibacterial activity. | [128] |
| Co0.5Fe0.5Fe2O4 Nanozyme | Ciprofloxacin, Azithromycin, Levofloxacin, Moxifloxacin, Amoxicillin, Metronidazole | pH 7, 0.5 mM H2O2, 15-min reaction, room temperature | Achieved near-complete removal of all six antibiotics. | [129] |
The Scientist's Toolkit: Key Research Reagents & Materials
Table 3: Essential Materials for Sol-Gel Synthesis and Bio-Photocatalytic Assessment
| Item Name | Specification/Example | Primary Function in Protocol |
|---|---|---|
| Metal Alkoxide Precursors | Titanium(IV) isopropoxide (TTiP), Tetraethyl orthosilicate (TEOS) | Primary source of metal oxide network formers in the sol-gel process. |
| Dopant Salts | Iron(III) nitrate nonahydrate, Niobium(V) ethoxide | Introduces dopant cations to modify band structure and enhance visible-light activity. |
| Solvent | Absolute Ethanol, 2-Methoxyethanol | Liquid medium for precursor dissolution and reaction control. |
| Chelating Agent | Citric Acid monohydrate | Controls hydrolysis and condensation rates; improves precursor stability and homogeneity. |
| Catalyst for Hydrolysis | Nitric Acid (HNO3), Ammonia (NH3) | Modifies pH to catalyze hydrolysis and condensation reactions. |
| Model Pharmaceutical Pollutants | Tetracycline Hydrochloride, Amoxicillin | Target compounds for evaluating photocatalytic degradation efficiency. |
| Model Organic Dyes | Methylene Blue, Rhodamine B | Benchmark pollutants for initial photocatalytic activity tests. |
| Bacterial Strain | E. coli ATCC 25922, S. aureus ATCC 25923 | Model organisms for standardizing antibacterial activity assays. |
| Culture Media | Mueller-Hinton Broth (MHB), Agar | Supports bacterial growth for antibacterial susceptibility testing. |
| Light Source | UVC Lamp (6 W, 254 nm), Solar Simulator | Provides photon energy to activate the photocatalyst. |
Within the broader context of sol-gel method research for metal oxide photocatalyst synthesis, assessing long-term stability and reusability is critical for transitioning laboratory innovations into practical, economically viable applications. Sol-gel synthesis enables the creation of metal oxide nanostructures with tailored compositional homogeneity, morphological control, and enhanced physicochemical properties [3] [17]. However, the true measure of a photocatalyst's potential lies in its ability to maintain performance integrity over multiple operational cycles without significant activity loss or structural degradation. Key failure modes include photocatalytic activity decay, often due to surface poisoning or catalyst leaching; structural instability, such as phase transformation or nanoparticle aggregation; and metal ion leaching from the catalyst framework into the solution, which raises secondary pollution concerns [21] [104]. This protocol establishes standardized methodologies for evaluating cycle performance and metal leaching in sol-gel-derived metal oxide photocatalysts, providing a framework for comparing material durability across different studies and accelerating the development of commercially feasible photocatalytic systems.
Quantitative Stability and Reusability Data from Literature
Table 1: Documented Cycle Performance of Sol-Gel Derived Photocatalysts
| Photocatalyst Composition | Target Pollutant | Initial Efficiency (%) | Cycles Tested | Final Efficiency (%) | Efficiency Retention (%) | Key Stability Observation | Source |
|---|---|---|---|---|---|---|---|
| Ce-TiOâ‚‚ Films (0.08 wt% Ce) | Ciprofloxacin | ~90 (Solar) | 3 | ~87 (Solar) | ~96.7 | Photocatalytic efficiency change <3%; excellent structural stability | [104] |
| Fe₃O₄–SiO₂–EN/ZnAl-LDH (FSEZAL) | Penicillin G | 100 (UV/Visible) | 5 | 94.3 (UV/Visible) | 94.3 | Slight decrease attributed to minor mass loss during recovery; maintained structural integrity | [130] |
| TiO₂–SiO₂ Mixed Oxide | Rhodamine B | ~92 (Initial) | - | - | - | Demonstrated concentration-dependent biological activities | [33] |
Table 2: Metal Leaching and Structural Characteristics
| Photocatalyst Composition | Analysis Technique | Metal Leaching Level | Structural Feature Post-Cycling | Surface Area/Porosity | Source |
|---|---|---|---|---|---|
| Fe₃O₄–SiO₂–EN/ZnAl-LDH (FSEZAL) | Not specified (Implied minimal) | Minimal (Inferred from high stability) | Core-shell structure enhances stability; prevents Fe₃O₄ aggregation | High surface area (28.67 m²/g); Mesoporous (1.64 nm pore size) | [130] |
| Ce-TiOâ‚‚ Films | XRD, DRS | Not quantified | Retained anatase phase; Suppressed electron-hole recombination | - | [104] |
| ZnO Nanoparticles | SEM, PL, XRD | - | Solvent type (ethanol) influenced morphology, reduced recombination | - | [17] |
Experimental Protocols for Stability Assessment
Protocol for Photocatalytic Cycle Performance Testing
Principle: This method evaluates the retention of photocatalytic degradation efficiency over multiple use cycles, simulating long-term operational conditions. The protocol assesses the catalyst's robustness against deactivation mechanisms like surface fouling, active site poisoning, and structural deterioration.
Materials and Equipment:
- Synthesized sol-gel photocatalyst (immobilized films or powdered form)
- Target pollutant stock solution (e.g., antibiotic, dye)
- Photocatalytic reactor system with controlled light source (UV, visible, or solar simulator)
- Magnetic stirrer or recirculation pump
- UV-Vis spectrophotometer or HPLC system for concentration analysis
- Centrifuge (for powder catalyst separation)
- Drying oven
Procedure:
- Baseline Performance Establishment: Conduct the initial photocatalytic degradation experiment under optimized, fixed conditions (catalyst loading, pollutant concentration, pH, light intensity, temperature, and reaction duration). Determine the initial degradation efficiency (η₀) using the formula: η₀ = (C₀ - Cₑ) / C₀ × 100%, where C₀ is the initial concentration and Cₑ is the concentration after the treatment cycle [104] [130].
- Catalyst Recovery:
- For immobilized films: Carefully remove the film from the reactor after each cycle, rinse gently with deionized water to remove loose surface residues, and air-dry at moderate temperature (e.g., 60-80°C) before reuse [104].
- For powdered catalysts: After each cycle, separate the catalyst from the suspension via centrifugation, magnetic separation (if magnetic composite), or filtration. Wash the recovered catalyst with deionized water and dry in an oven (e.g., 100°C) to constant weight [130].
- Reuse Cycling: Reintroduce the recovered catalyst into a fresh batch of pollutant solution at identical initial concentration and volume. Repeat the photocatalytic degradation process under the same conditions as the baseline.
- Efficiency Monitoring: Measure the degradation efficiency (ηₙ) after each cycle (n). A stable catalyst will show minimal reduction in ηₙ over successive cycles.
- Post-Cycling Characterization: After the final cycle, characterize the catalyst using techniques such as XRD (phase stability), SEM (morphological changes), FT-IR (functional group integrity), and BET (surface area changes) to correlate performance loss with structural alterations [17] [104] [130].
Figure 1: Workflow for photocatalytic cycle performance testing
Protocol for Metal Leaching Analysis
Principle: This procedure quantifies the extent of metal ion release from the photocatalyst into the aqueous solution during operation. Leaching compromises catalytic activity, alters surface composition, and poses environmental risks, making its assessment crucial.
Materials and Equipment:
- Photocatalyst sample
- Aqueous reaction medium (e.g., deionized water, buffered solution)
- Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) or Mass Spectrometry (ICP-MS)
- Centrifugation filters (0.45 µm or smaller pore size, if needed)
- Acid digestion vessels
- Ultrapure nitric acid
Procedure:
- Leachate Collection: After the photocatalytic reaction, separate the catalyst from the aqueous phase thoroughly using high-speed centrifugation or membrane filtration (0.45 µm or 0.22 µm pore size) to ensure no catalyst particles remain in the liquid [130].
- Sample Preservation: Acidify the collected leachate (e.g., with 2% ultrapure nitric acid) to prevent adsorption of metal ions onto container walls and refrigerate if not analyzed immediately.
- Analysis via ICP-OES/MS:
- Direct Analysis: For expected high concentrations, analyze the filtered leachate directly after appropriate dilution.
- Digestion for Total Leachable Content: To determine the total leachable metal content, digest a known amount of the fresh catalyst in concentrated nitric acid using a microwave digester or hot block. Analyze the digestate after dilution. This provides a reference for the maximum potential leaching [131].
- Quantification:
- Prepare a series of standard solutions of the target metal ions (e.g., Ti, Zn, Ce, Fe) for calibration.
- Analyze the leachate samples and quantify the concentration of each metal ion.
- Calculate the leaching extent as a percentage of the total metal content in the catalyst: Leaching % = (Mass of metal in leachate / Total mass of metal in catalyst used) × 100%.
- Correlation with Performance: Correlate the leaching extent observed after the first cycle and any increase over subsequent cycles with the decay in photocatalytic activity. Significant leaching often leads to a steady decline in performance.
Figure 2: Metal leaching analysis workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Stability and Leaching Studies
| Category | Item | Function in Protocol | Example/Note |
|---|---|---|---|
| Target Pollutants | Ciprofloxacin | Model antibiotic pollutant for degradation stability tests | Used with Ce-TiOâ‚‚ films [104] |
| Penicillin G (PNG) | Model antibiotic to assess catalytic activity retention | Degraded by FSEZAL nanocomposite [130] | |
| Methylene Blue (MB) / Rhodamine B (RhB) | Model dye pollutants for initial activity screening | Used for TiOâ‚‚-SiOâ‚‚ and ZnO efficiency tests [33] [17] | |
| Catalyst Components | Titanium(IV) Isopropoxide (TIP) | Common Ti precursor for sol-gel TiOâ‚‚-based catalysts | Used in Ce-TiOâ‚‚ film synthesis [104] |
| Cerium(III) Nitrate Hexahydrate | Dopant precursor to enhance visible light response & stability | Dopant for TiOâ‚‚ films [104] | |
| Zinc Acetate Dihydrate | Zn precursor for ZnO-based catalysts | Forms zinc oxalate intermediate [17] | |
| Tetraethyl Orthosilicate (TEOS) | SiO₂ precursor for creating protective/mesoporous matrices | Used in Fe₃O₄–SiO₂ core-shell structures [130] | |
| Analytical Tools | ICP-OES/MS Standard Solutions | Quantification of metal ion concentrations in leachates | Critical for leaching analysis [131] |
| XRD Analysis Software | Phase identification and crystallinity tracking post-cycling | Monitors phase stability (e.g., anatase retention) [104] | |
| SEM/TEM | Morphological characterization before/after cycling | Assesses particle aggregation, structural integrity [33] [130] | |
| Surface Area Analyzer (BET) | Measures surface area and pore volume changes | Confirms mesoporous structure stability [130] |
The structured protocols for cycle performance testing and metal leaching analysis presented herein provide a standardized framework for evaluating the operational longevity of sol-gel-derived metal oxide photocatalysts. The empirical data summarized from recent studies demonstrates that carefully engineered sol-gel materials, such as doped TiOâ‚‚ films and magnetic nanocomposites, can achieve excellent stability over multiple cycles with minimal efficiency loss and metal leaching. Integrating these assessments into the standard characterization pipeline is paramount for developing photocatalysts that are not only highly active but also durable and environmentally benign, thereby bridging the gap between laboratory-scale innovation and real-world water treatment applications.
Conclusion
The sol-gel method offers unparalleled versatility in designing metal oxide photocatalysts with precise control over composition, morphology, and optical properties. By leveraging heterojunction engineering, strategic doping, and optimized synthesis protocols, researchers can overcome fundamental limitations of traditional photocatalysts, particularly poor visible-light response and rapid charge recombination. The demonstrated efficacy of sol-gel-derived composites in degrading complex organic molecules, including pharmaceuticals and dyes, positions this synthesis approach as a cornerstone for developing advanced environmental remediation technologies and novel biomedical applications. Future research should focus on green synthesis routes, machine-learning-assisted optimization, integration with biomedical platforms for therapeutic applications, and scaling advanced microwave-assisted techniques for industrial production to bridge laboratory innovation with clinical and commercial implementation.
References
- 1. Sol-Gel Processing - an overview [https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/sol-gel-processing]
- 2. Sol–gel process [https://en.wikipedia.org/wiki/Sol%E2%80%93gel_process]
- 3. Sol–Gel-Synthesized Metal Oxide Nanostructures [https://www.mdpi.com/2310-2861/11/8/657]
- 4. Sol-gel Syntheses of Photocatalysts for the Removal ... [https://www.mdpi.com/2079-4991/9/1/126]
- 5. “Traditional†Sol-Gel Chemistry as a Powerful Tool for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6416638/]
- 6. Sol–gel synthesis and characterization of ZnO–SiO 2 ... [https://pubs.rsc.org/en/content/articlehtml/2025/na/d5na00612k]
- 7. Review Tunable photocatalytic activity of metal oxides [https://www.sciencedirect.com/science/article/abs/pii/S0301479725036734]
- 8. TiO2 photocatalysis: Design and applications [https://www.sciencedirect.com/science/article/pii/S1389556712000421]
- 9. A Review of Photocatalysts Prepared by Sol-Gel Method ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2904920/]
- 10. Colloidal sol-gel: A powerful low-temperature aqueous ... [https://www.sciencedirect.com/science/article/pii/S2666539521001462]
- 11. Synthesis and Characterization of a Novel Sol–Gel- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11990163/]
- 12. Low-temperature sol–gel methods for the integration of ... [https://link.springer.com/article/10.1007/s10971-023-06065-2]
- 13. Enhancing Optical Properties and Cost-Effectiveness of Sol ... [https://www.mdpi.com/2227-9717/13/7/1988]
- 14. Sol–Gel Synthesis and Multi-Technique Characterization of ... [https://www.mdpi.com/2073-4360/17/20/2767]
- 15. Recent trends of titania (TiO2) based materials [https://jksus.org/recent-trends-of-titania-tio2-based-materials-a-review-on-synthetic-approaches-and-potential-applications/]
- 16. Tailored morphology of indium-doped titania via sol-gel ... [https://www.sciencedirect.com/science/article/pii/S027288422501003X]
- 17. Enhanced photocatalytic efficiency of eco-friendly synthesized ... [https://pubs.rsc.org/en/content/articlehtml/2025/ma/d5ma00026b]
- 18. Synthesis, Optical Properties and Photocatalytic Testing of ... [https://www.mdpi.com/2673-3269/6/2/22]
- 19. Microstructure and Photocatalytic Performance of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12469563/]
- 20. Band gap engineering of tungsten oxide-based ... [https://www.sciencedirect.com/science/article/abs/pii/S1389556724000315]
- 21. Interfacially engineered metal oxide nanocomposites for ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d4ra08780a]
- 22. Charge carrier dynamics in metal oxides | DYNAMOX | Project [https://cordis.europa.eu/project/id/695197]
- 23. Enhanced charge carrier separation and transfer in gC 3 N 4 ... [https://pubs.rsc.org/en/content/articlehtml/2025/cp/d5cp03846d?page=search]
- 24. Applications of Metal/Mixed Metal Oxides as Photocatalyst [http://www.orientjchem.org/vol32no4/applications-of-metalmixed-metal-oxides-as-photocatalyst-a-review/]
- 25. Ti–Fe mixed oxides as photocatalysts in the generation of ... [https://www.sciencedirect.com/science/article/abs/pii/S0360319922017517]
- 26. Green Sol-gel Synthesis of TiO2, SiO2, and ZnO Based ... [https://re.public.polimi.it/handle/11311/1299009]
- 27. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11085299/]
- 28. Nanosized TiO2 - Based Mixed Oxide Films [http://article.sapub.org/10.5923.j.ijme.20130306.02.html]
- 29. Synthesis and characterization of TiO2/ZnO/SiO2 ... [https://www.sciencedirect.com/science/article/abs/pii/S0921510724005646]
- 30. Sol–gel synthesis and characterization of ZnO–SiO2 ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12505225/]
- 31. Study of the Interaction of Ti–Zn as a Mixed Oxide at ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9229482/]
- 32. Synthesizing and Evaluating the Photocatalytic and ... [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2021.738969/full]
- 33. Sol–Gel TiO2–SiO2 Mixed Oxides as Dual-Functional ... [https://www.sciencedirect.com/science/article/pii/S246821792500190X]
- 34. The evolution of 'sol–gel' chemistry as a technique for ... [https://pubs.rsc.org/en/content/articlehtml/2016/mh/c5mh00260e]
- 35. Sol–Gel Technology Applied to Materials Science [https://pmc.ncbi.nlm.nih.gov/articles/PMC10817290/]
- 36. Relationship between morphology, porosity, and the ... [https://www.sciencedirect.com/science/article/pii/S036631751930086X]
- 37. Impact on the Photocatalytic Dye Degradation of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10157689/]
- 38. A new way to tune photocatalytic activity, surface ... [https://link.springer.com/article/10.1007/s10971-024-06534-2]
- 39. Key Steps in the Sol-Gel Process for Nanoparticle Synthesis [https://www.azonano.com/article.aspx?ArticleID=6857]
- 40. Sol–Gel Synthesis and Comprehensive Study of Structural, ... [https://www.mdpi.com/2310-2861/11/6/450]
- 41. Recent advances in sol–gel synthesis of monolithic silica ... [https://www.sciencedirect.com/science/article/pii/S2187076413000225]
- 42. Precursor reaction pathway leading to BiFeO 3 formation [https://pubs.rsc.org/en/content/articlehtml/2025/dd/d5dd00160a]
- 43. Microwave assisted sol gel synthesis of Fe2O3@TiO2 core ... [https://www.sciencedirect.com/science/article/abs/pii/S092188312400390X]
- 44. Microwave-Assisted Vacuum Synthesis of TiO2 ... - PMC - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC8746694/]
- 45. Sol-gel hydrothermal synthesis of lead-free BT ... [https://www.sciencedirect.com/science/article/abs/pii/S0169433224019561]
- 46. Facile Hydrothermal Assisted Basic Catalyzed Sol Gel ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11675958/]
- 47. Simultaneous microwave-ultrasound irradiation of Ag/TiO 2 ... [https://www.sciencedirect.com/science/article/abs/pii/S0019452225005953]
- 48. HDTMS Fabricated via a Two-step Spray Method [https://www.sciencedirect.com/science/article/abs/pii/S0927775725026317]
- 49. Synthesis and characterization of TiO / 2 nano SiO for... 2 composites [https://link.springer.com/article/10.1007/s13204-012-0060-5]
- 50. Study on photocatalytic and antibacterial ability of TiO and... 2 [http://jase.tku.edu.tw/articles/jase-202503-28-03-0004]
- 51. Morphology dependent antibacterial activity of zinc oxide ... [https://www.nature.com/articles/s41598-025-29075-2]
- 52. Multifunctional transition metal oxide/graphene oxide ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra04806k?page=search]
- 53. Heterojunction photocatalysts: where are they headed? [https://pubs.rsc.org/en/content/articlehtml/2025/lf/d5lf00037h]
- 54. A sol-gel fabrication, characterization, and boosted ... [https://www.sciencedirect.com/science/article/pii/S2667022425001495]
- 55. A Comprehensive Review of Z-, S-, R-, and C-scheme ... [https://www.sciencedirect.com/science/article/abs/pii/S0925838825058062]
- 56. Modulation of Z-scheme photocatalysts for pharmaceuticals ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9422400/]
- 57. Facile fabrication of Zn 0.6 Cd 0.4 S/ZnO/g-C 3 N 4 dual Z- ... [https://www.nature.com/articles/s41598-025-01198-6]
- 58. Exploring the diverse applications of sol–gel synthesized ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11393273/]
- 59. Band Gap Tuning in Transition Metal and Rare-Earth-Ion ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9824300/]
- 60. Phase transition and bandgap modulation in TiO 2 ... [https://www.nature.com/articles/s41598-025-07000-x]
- 61. Transition metal dopants modulate the band gap and ... [https://www.sciencedirect.com/science/article/pii/S2949822825000681]
- 62. Non-metal doped ZnO and TiO2 photocatalysts for visible ... [https://www.sciencedirect.com/science/article/pii/S2950648425000185]
- 63. Stability of non-metal dopants to tune the photo-absorption ... [https://www.nature.com/articles/s41598-019-47710-7]
- 64. Transition metal doping effects on the structural ... [https://link.springer.com/article/10.1007/s00339-025-08942-9]
- 65. Sol-gel Syntheses of Photocatalysts for the Removal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6358872/]
- 66. Advanced photocatalytic degradation of POPs and other ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra04336k?page=search]
- 67. Experimental and computational study of metal oxide ... [https://pubs.rsc.org/en/content/articlehtml/2023/ra/d3ra01505j]
- 68. CeO 2 sol–gel photocatalysts: Effect of the annealing ... [https://www.sciencedirect.com/science/article/abs/pii/S1010603010004636]
- 69. Heterogeneous photocatalytic degradation of selected ... [https://pubs.rsc.org/en/content/articlelanding/2025/ra/d5ra03247d]
- 70. Photocatalytic sol-gel/P25 TiO2 coatings for water treatment [https://www.sciencedirect.com/science/article/pii/S027288422203382X]
- 71. Photocatalytic degradation of methylene blue dye by ZnO ... [https://www.nature.com/articles/s41598-024-76938-1]
- 72. Sol-Gel Synthesis of Fe2o3-Doped Tio2 Photocatalyst for ... [http://www.orientjchem.org/vol33no4/sol-gel-synthesis-of-fe2o3-doped-tio2-photocatalyst-for-optimized-photocatalytic-degradation-of-24-dichlorophenoxyacetic-acid/]
- 73. Perspective Chapter: Sol-Gel Science and Technology in ... [https://www.intechopen.com/chapters/86894]
- 74. Sol-Gel Processing - an overview [https://www.sciencedirect.com/topics/materials-science/sol-gel-processing]
- 75. Interfacially engineered metal oxide nanocomposites for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12068376/]
- 76. Recent developments on metal oxide-based photocatalysts ... [https://www.sciencedirect.com/science/article/abs/pii/S295028452500027X]
- 77. Experimental and computational study of metal oxide ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10278095/]
- 78. Semiconductor photocatalysts for hydrogen evolution: critical role of... [https://pubs.rsc.org/en/content/articlehtml/2025/cc/d5cc04459f]
- 79. Metal-centred states control carrier lifetimes in transition ... [https://www.nature.com/articles/s41557-025-01868-y]
- 80. Enhanced photocatalytic activity of Se-doped TiO 2 under ... [https://www.nature.com/articles/s41598-018-27135-4]
- 81. Defect engineering for enhanced optical and photocatalytic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10556056/]
- 82. Defect-enhanced visible-light photocatalysis in α–Fe2O3 ... [https://www.sciencedirect.com/science/article/abs/pii/S0169433225021361]
- 83. Harnessing visible light: Advanced photocatalytic ... [https://www.sciencedirect.com/science/article/pii/S1385894725057870]
- 84. Sol gel graphene/TiO2 nanoparticles for the photocatalytic- ... [https://www.sciencedirect.com/science/article/pii/S0926337318309883]
- 85. Advanced microwave synthesis strategies for innovative ... [https://www.sciencedirect.com/science/article/pii/S2468025724003194]
- 86. Microwave-assisted synthesis of nanomaterials: a green ... [https://pubs.rsc.org/en/content/articlehtml/2025/su/d5su00584a]
- 87. Overcoming Scaling Challenges in Sol–Gel Synthesis [https://www.mdpi.com/3042-5697/1/2/6]
- 88. Microwave-Assisted Synthesis of Iron-Based Aerogels with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10580704/]
- 89. Sol–gel synthesis method [https://bio-protocol.org/exchange/minidetail?id=16807250&type=30]
- 90. Influence of Synthesis and Optimization Parameters on NO ... [https://www.sciencedirect.com/science/article/abs/pii/S0022369725004573]
- 91. Unraveling the impact of microwave-assisted techniques in ... [https://www.nature.com/articles/s41598-023-51078-0]
- 92. Effect of thermal treatments on high surface area anatase ... [https://pubs.rsc.org/en/content/articlehtml/2025/cp/d5cp01731a]
- 93. A feasible strategy to balance the crystallinity and specific ... [https://www.nature.com/articles/srep46424]
- 94. Mesoporous glass-ceramic powder obtained by thermal ... [https://www.sciencedirect.com/science/article/abs/pii/S0272884225043901]
- 95. Effect of hydrothermal treatment on structural and ... [https://www.sciencedirect.com/science/article/abs/pii/S0926860X11006284]
- 96. Comparison of sol-gel and co-precipitation methods on the ... [https://link.springer.com/article/10.1007/s40436-013-0018-1]
- 97. Comparative Study of MgO Nanopowders Prepared by ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10453559/]
- 98. Toward Sustainable PFAS Elimination: Engineering TiO 2 ... [https://www.sciencedirect.com/science/article/pii/S2468217925001947]
- 99. Carbon Nitride Gels: Synthesis, Modification, and Water ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12470228/]
- 100. Review on the synergistic effect of adsorption and ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra01082a?page=search]
- 101. Advances in Composite Photocatalysts for Efficient ... [https://www.mdpi.com/2073-4344/15/9/893]
- 102. Design, synthesis, and optimization of a novel ternary ... [https://www.nature.com/articles/s41598-025-93478-4]
- 103. Sol–gel auto-combustion synthesis of a novel ternary ... [https://link.springer.com/article/10.1007/s13201-025-02549-4]
- 104. Synthesis, Characterization, and Photocatalytic Properties ... [https://www.mdpi.com/2227-9717/12/6/1144]
- 105. Sol-gel synthesis, characterization, and electrochemical ... [https://link.springer.com/article/10.1007/s10971-023-06260-1]
- 106. Sol–Gel Synthesis and Characterization of a Quaternary ... [https://www.mdpi.com/1996-1944/14/16/4515]
- 107. Gels: Synthesis, Characterization and Applications in High ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10138156/]
- 108. FT-IR Characterization of Antimicrobial Hybrid Materials ... [https://www.mdpi.com/2076-3417/10/3/1180]
- 109. Characterization of Hybrid Materials Prepared by Sol-Gel ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8038627/]
- 110. Photocatalytic apparent quantum yield exceeding 100% ... [https://www.sciencedirect.com/science/article/abs/pii/S0169433225003526]
- 111. A Review on the Use of Metal Oxide-Based ... [https://www.mdpi.com/2305-6304/11/8/658]
- 112. Novel sol-gel synthesis of TiO 2 /BiPO 4 composite for ... [https://www.sciencedirect.com/science/article/abs/pii/S2214714425001709]
- 113. Recent developments in the use of metal oxides for ... [https://www.sciencedirect.com/science/article/pii/S2468519420301403]
- 114. The adsorption/photocatalytic degradation kinetics of ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894725003250]
- 115. A modified sol-gel synthesis protocol for high-quality ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12483647/]
- 116. A comparative study for optimizing photocatalytic activity of ... [https://www.nature.com/articles/s41598-024-77752-5]
- 117. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications [https://pmc.ncbi.nlm.nih.gov/articles/PMC12073315/]
- 118. Enhanced photocatalytic activity by structural defect of ZnO ... [https://www.sciencedirect.com/science/article/abs/pii/S0272884225035059]
- 119. Exploring the effective parameters on the photocatalytic ... [https://www.sciencedirect.com/science/article/pii/S1026918525000484?dgcid=rss_sd_all]
- 120. Improving Photocatalytic Hydrogen Production with Sol ... [https://www.mdpi.com/1996-1073/17/23/5830]
- 121. Tunable photocatalytic activity of metal oxides [https://pubmed.ncbi.nlm.nih.gov/41175492/]
- 122. Photocatalytic degradation of methylene blue, rhodamine B ... [https://www.sciencedirect.com/science/article/pii/S138770032200572X]
- 123. Natural Sunlight Driven Photocatalytic Degradation of ... [https://www.mdpi.com/2079-4991/15/14/1138]
- 124. Photocatalytic degradation of rhodamine B and methylene ... [https://pubmed.ncbi.nlm.nih.gov/40220157/]
- 125. Recent prospects, challenges and advancements of ... [https://www.oaepublish.com/articles/wecn.2025.03]
- 126. Optical, Photocatalytic, and Antibacterial Properties of Sol- ... [https://www.mdpi.com/2073-4441/17/14/2072]
- 127. The Antimicrobial Effect and ROS Redox Activity of Nb2O5 ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12469671/]
- 128. Degradation and Loss of Antibacterial Activity ... [https://www.mdpi.com/2073-4344/8/6/222]
- 129. Exploration of degradation pathways of six antibiotics using ... [https://www.nature.com/articles/s41598-025-23740-2]
- 130. Enhanced photocatalytic degradation of penicillin G using ... [https://www.nature.com/articles/s41598-025-17327-0]
- 131. Metal Oxides Nanoparticles: General Structural Description ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10096196/]